The road to new aortic valve disease treatment: Innovations in 3D models and drug delivery
Calcific Aortic Valve Disease (CAVD) resulting in aortic stenosis is a common heart disease that affects 25% of people over 65 years old, causing 17,000 deaths per year in the USA. The aortic valve regulates blood flow from the heart to the rest of the body. In patients with CAVD, calcium mineral deposits within the aortic valve make the valve thicker and stiffer. This prevents the valve from opening and closing properly, resulting in lower blood supply to the body, making it difficult for patients to do daily routine activities, such as walking up the stairs, due to quick exhaustion. Without aortic valve replacement via valve-in-valve procedure or open-heart surgery these patients die within a few years of diagnosis. During surgery the diseased valve is replaced with a prosthetic valve. Prosthetic valves can be mechanical (made of metal) or biological (made of a valve-like tissue of a cow or a pig). Valve replacements are costly, but it is the only option for patients. The number of surgeries in the USA is around 275,000 per year and it is expected to increase three-fold by 2050. The authors of this paper believe that there must be a better way to treat this devastating disease.

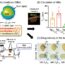
Fig. 1. Density-dependent color scanning electron microscopy (DDC-SEM). Mineral is presented in orange and extracellular matrix is presented in green.
为了研究这种疾病必须代表官方manbetx手机版licated in a laboratory. This paper explains that to replicate the disease, researchers take the heart valves from patients who have undergone surgery and grow the cells from these valves in the lab, allowing the scientists to study the disease in a controlled environment. The researchers make sure that the cells behave the same way in the lab as they would in the patients by mimicking the valve tissue the cells are normally in. A 3D CAVD model is made by mixing the cells with a hydrogel that consists of fibres of the same proteins that make up the valve and printing it layer by layer to create a 3D replica. This 3D CAVD model has the same structure as the valve of the patients, and has three major benefits. First, researchers can use this 3D model to look at how the disease develops over time. Second, it can be used to discover and test new kinds of medication that can slow down, or even prevent the disease. Third, it can be used to verify new ways to bring the medication into the valve.

Fig. 2. 3D-bioprinted CAVD model – Isolated human aortic Valve Interstitial Cells are suspended in a hydrogel pre-polymer solution with photo-initiator cross-linking agent (A).Three distinct layers are bioprinted and cross-linked using UV light (B). Exposure to osteogenic medium activates the quiescent VICs to differentiate into activated VICs and osteogenic VICs (C), leading to the formation of microcalcifications, thereby mimicking CAVD progression.
The paper continues to explain that to study disease development, the pathways that are activated inside the heart valve cells during the disease are mapped and key components of this pathway are identified. Then different types of medication are identified that can potentially block or stimulate these key components. This review highlights the potential of small pieces of genetic material called microRNA that have the potential to function as medication. Depending on the type of medication that is most effective, a fitting drug delivery device will be engineered to ensure that the medication gets to where it needs to go inside the body. This last section elaborates on chemical characteristics and modifications that the microRNA needs to have and undergo in order to ensure that they will arrive safely at their destination. Finally, future and clinical perspectives are noted to highlight how these types of 3D disease models and drug delivery devices have the potential to be used in treating different diseases and how they can help speeding up the translation of scientific work to clinical solutions.
Casper van der Ven1,2,3,Elena Aikawa1,4
1Center for Excellence in Vascular Biology, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA
2David H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, Massachusetts, USA
3Regenerative Medicine Center Utrecht, University Medical Center Utrecht, The Netherlands
4Center for Interdisciplinary Cardiovascular Sciences, Cardiovascular Medicine,
Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA
Publication
In vitro 3D model and miRNA drug delivery to target calcific aortic valve disease.
van der Ven CF, Wu PJ, Tibbitt MW, van Mil A, Sluijter JP, Langer R, Aikawa E
Clin Sci (Lond). 2017 Feb












Leave a Reply
You must belogged into post a comment.