Simple method for measuring important components of cannabis in blood
The first recorded use ofcannabis sativa, commonly called hemp, was by the ancient Chinese as early as 4000 BC. It was used as a fiber source for textiles, ropes, and paper. In addition, medical and entertainment uses of cannabis were well documented by Indians, Chinese, and Assyrians centuries before the Christian era. Cannabis is a unique source of around 60 chemical compounds collectively known as cannabinoids. There has been increased interest in the medical use of cannabinoids in recent years, particularly in the predominant natural cannabinoids, cannabidiol (CBD) and Δ9-tetrahydrocannabinol (THC). This was supported by evidence-based studies and testimony from patients who benefited from the use of medical cannabis. Subsequently, increasing public and political pressures supported the legalisation of cannabis for medical use. At present, cannabis is legalized for medical use in 23 states of the US, as well as in Canada, the Netherlands, and Israel. In addition, there are other states and countries which are currently considering the legalization of medical cannabis, such as Australia and New Zealand.

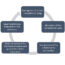
Fig. 1. Schematic representation of the experimental set-up of the preliminary pharmacokinetic study following IV bolus administration of 5 mg/kg cannabidiol (CBD) and Δ9-tetrahydrocannabinol (THC) to rats (n=2). (1) Blood sampling from the fitted cannula. (2) Chromatogram shows CBD, THC, and the internal standard DDT following plasma analysis by HPLC. (3) Data analysis by the pharmacokinetic software. (4) Plasma concentration-time profiles of CBD and THC in rats.
Researches have shown that both CBD and THC have a wide range of therapeutic activities. A significant number of these researches were using rat animal models of the diseases in question. Therefore, the aim of the present study was to develop a simple method for the measurement of CBD and THC in rat plasma using a technique commonly available in research laboratories. This technique is the high-performance liquid chromatography (HPLC) coupled with ultraviolet (UV) detection. In this system, CBD and THC can be detected as bands (peaks) on a chromatogram.
Plasma samples were first treated with the organic solvent acetonitrile to clean-up the high protein contents. Next, n-hexane (another organic solvent) was used to extract CBD and THC. Extracted samples were then injected in the HPLC system for analysis.
The current method was validated in accordance with the Food and Drug Administration (FDA) Guidance or Bioanalytical Method Validation in the following terms:
- Selectivity: the ability of the developed method to measure and differentiate CBD and THC form other components in rat plasma.
- Precision: the reproducibility of CBD and THC measurements obtained from multiple sampling.
- Accuracy: the closeness of the measured concentrations of CBD and THC to the true concentrations.
- Sensitivity: The lowest amount of CBD or THC in a sample that can be quantified with appropriate precision and accuracy.
To show the suitability of the method for studies in rats, the assay was applied to a preliminary pharmacokinetic study (the movement of drug into, though, and out of the body) following intravenous administration of 5 mg/kg CBD or THC. In this study, a small tube (cannula) was inserted in the right jugular vein of the rats for drug administration. Blood was sampled from the same cannula before dosing, and at predetermined time points following the administration of cannabinoids. Plasma was separated from blood by centrifugation and processed as described above for analysis (Fig. 1).
The results of the current study showed that the developed method has good selectivity since both CBD and THC peaks were well separated and distinguished from other peaks in the sample (Fig. 1). In addition, precision and accuracy for CBD and THC were within the acceptable limits for all samples. In fact, the method was found to be suitable for the quantification of concentrations as low as 10 ng/mL for both cannabinoids.
In conclusion, a simple, sensitive, and cost-efficient HPLC-UV method for the simultaneous determination of CBD and THC has been successfully developed, validated and applied to a pharmacokinetic study in rats.
Publication
Development of a simple and sensitive HPLC-UV method for the simultaneous determination of cannabidiol and Δ(9)-tetrahydrocannabinol in rat plasma.
Zgair A, Wong JC, Sabri A, Fischer PM, Barrett DA, Constantinescu CS, Gershkovich P
J Pharm Biomed Anal. 2015 Oct 10











Leave a Reply
You must belogged into post a comment.