Phospholipases have a second job, regulating their G proteins
Can we make a weak heart healthy? Can we prevent decline in memory and cognitive ability? The answer may depend on a class of enzymes, phospholipase C (PLC), and their under-investigated ability to directly regulate their specific G proteins. G proteins, aka GTP-binding proteins, are cellular switches for a ligand activated cell-surface receptor. G proteins control the activity of effectors; proteins which regulate cell activity and response.
The G protein is switched- on by the receptor driven release of bound-GDP. Rapid replacement with GTP creates the active species, G protein-GTP. The G protein is switched- off by an intrinsic GTPase, which hydrolyzes the bound GTP to GDP to increase fraction of G protein-GDP. This cycling of G proteins between an active and inactive state is termed the GTPase cycle. The GTPase cycle is central to the switch function of the G protein.

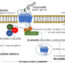
Fig. 1. Model describes auto-amplification of signaling by a specific G protein through synergistic lipase/ G protein regulating activity of PLC, and modulation by the binding of phosphatidic acid (PA).
G protein stimulation of the large PLC family results in the hydrolysis of membrane phospholipid to release products that signal changes in cellular activity. Different subfamilies of PLC function as effectors for different G proteins which control different responses. PLC is traditionally viewed as a major G protein effector. But is there more to tell about these hard working lipases? The answer appears to be yes. Select members of the PLC family have G protein regulating activity. They can directly regulate their activating G protein.
Why is this significant? G proteins are important to human health as evidenced by the large number of pharmaceuticals which target G protein signaling. Most strive to regulate response at the level of the receptor. However, receptors regulate many cellular processes. Therefore, manipulation of a specific response can be difficult. Undesired side effects result in low patient compliance.
In contrast, the direct targeting of specific G proteins offers an opportunity for more selective and efficacious therapies. A strategy is suggested with the finding that the G protein GTPase cycle is regulated by accessory proteins. GTPase activating proteins (GAP) accelerate GTP hydrolysis. Guanine nucleotide exchange factors (GEFs) catalyze the exchange of GTP for GDP. Therefore, manipulation of GAP and GEF activity is an approach to directly regulate the G protein.
Intriguingly, the PLC-β, PLC-δ, PLC-γ and PLC-ε subfamilies are G protein effectors which have been shown to have intrinsic GAP or GEF activity. The article reviews the evidence and discusses the possibility that the intrinsic GAP/GEF activity may allow these PLCs to perform a second job, functioning as positive regulators of their activating G protein.
在本质上,提出了PLC通信s activating G protein, manipulating the G protein GTPase cycle, to amplify G protein stimulation of PLC lipase activity (Fig.1). Regulation is specific to the PLC and G protein. Therefore, auto-amplification of a specific G protein-regulated response would occur by this mechanism.
Even more exciting is that observation that the lipase activity of these four PLC subfamilies is stimulated by an endogenous signaling molecule, phosphatidic acid (PA). Furthermore, PA amplifies G protein stimulated PLC-β lipase activity. A unique PA- binding domain within the G protein regulatory domain has been identified. Therefore, a possibility is that PA regulates synergistic lipase-G protein regulatory activity to control the level of auto-amplification. This hypothesis is supported by the knowledge that PA modulates specific GAPs and GEFs.
In conclusion, control of synergistic lipase-G protein regulatory activity by PA, represents a novel mechanism to modulate physiological response at the level of a G protein. A novel strategy to target specific G proteins is suggested.
Publication
Regulating G protein activity by lipase-independent functions of phospholipase C.
Litosch I
Life Sci. 2015 Sep 15












Leave a Reply
You must belogged into post a comment.