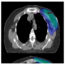
Palpable melanoma groin lymph node metastases
Melanoma is the most lethal type of skin cancer. In 2012 232,130 persons were diagnosed worldwide, and 55,488 estimated deaths occurred due to melanoma.
病人有明显的淋巴结tastasis in the groin area are a subgroup with a poor prognosis; at 5 years post diagnosis only 12 – 52% are alive. Currently, treatment for these patients consists of a therapeutic lymph node dissection. This can be a superficial groin dissection (SGD) including the superficial inguinal lymph nodes or a combined superficial and deep groin dissection (CGD) also including the deeper pelvic lymph nodes along the iliac artery and vein, and the obturator loge.
 There is ongoing debate whether this radical CGD is really necessary, or if a SGD would suffice. Morbidity may be more limited when performing SGD alone, and several studies have shown comparable local control rates and similar survival. However, if deep pelvic lymph node metastases are suspected at preoperative CT-scan or PET-CT scan, a CGD is warranted in order to remove all visible tumor in the pelvic area.
There is ongoing debate whether this radical CGD is really necessary, or if a SGD would suffice. Morbidity may be more limited when performing SGD alone, and several studies have shown comparable local control rates and similar survival. However, if deep pelvic lymph node metastases are suspected at preoperative CT-scan or PET-CT scan, a CGD is warranted in order to remove all visible tumor in the pelvic area.
This study aimed to analyze risk factors for presence of pelvic lymph node metastases in a cohort of patients with palpable melanoma lymph node metastases in the groin area. This could aid in the development of an algorithm for more selective surgery in the future.
A total of 209 patients were selected who underwent a CGD in one of four tertiary melanoma centers in the Netherlands between 1992–2013. Selection was based on presence of an adequate preoperative (PET) CT-scan and a detailed pathology report describing the number of removed lymph nodes. Analyzed risk factors included baseline and primary tumor characteristics, number of removed inguinal lymph nodes (including number of tumor positive lymph nodes), inguinal lymph node ratio (LNR = no. of tumor positive inguinal nodes divided by the total no. of removed inguinal nodes), and suspicious deep pelvic lymph nodes on preoperative imaging (CT or PET-CT-scan).
The median age of all patients was 57 years, 54% of patients were female. Median follow-up was 21 months (interquartile range (IQR, i.e. 25% and 75%) 11 – 46 months). Median Breslow thickness of the primary melanomas was 2.10mm (IQR 1.40 – 3.40mm), and 26% of all primary melanomas were ulcerated, (which is a poor prognostic factor). Tumor positive deep pelvic nodes were present in 35% of all patients.
Patients were divided into two groups based on presence or absence of tumor positive deep pelvic nodes. Patients without pelvic lymph node metastases had significantly fewer tumor positive inguinal nodes: median 1 positive inguinal node vs. median 3 positive inguinal nodes for patients with pelvic node metastases (p<0.001). The LNR was also significantly lower for patients without pelvic node metastases; median 0.15 vs. 0.33 for patients with pelvic nodal metastases (p<0.001).
A model was designed to predict absence of pelvic lymph node metastases based on the following: absence of suspicious pelvic nodes on preoperative imaging, number of positive inguinal lymph nodes, LNR, and presence/absence of extracapsular tumor extension. A combination of negative imaging, few positive inguinal lymph nodes, a low LNR and no extracapsular extension could accurately predict the absence of pelvic lymph node metastases in 84% of the patients selected for this model.
This study demonstrates that patients with negative imaging, few positive inguinal lymph nodes, no extracapsular tumor extension and a low LNR have a low risk of positive pelvic lymph node metastases and may safely undergo an SGD alone. The prediction model tested will be validated and may be used for future patients to select them for SGD safely.
Charlotte M.C. Oude Ophuis, MD
Dep. of Surgical Oncology, Erasmus MC Cancer Institute, Rotterdam, the Netherlands
Publication
Risk Factors for Positive Deep Pelvic Nodal Involvement in Patients with Palpable Groin Melanoma Metastases: Can the Extent of Surgery be Safely Minimized? : A Retrospective, Multicenter Cohort Study.
Oude Ophuis CM, van Akkooi AC, Hoekstra HJ, Bonenkamp JJ, van Wissen J, Niebling MG, de Wilt JH, van der Hiel B, van de Wiel B, Koljenović S, Grünhagen DJ, Verhoef C.
Ann Surg Oncol. 2015 May 27











Leave a Reply
You must belogged into post a comment.