A novel approach for the treatment of Juvenile Idiopathic Arthritis and possibly other autoimmune diseases.
Juvenile Idiopathic Arthritis (JIA) is a leading chronic autoimmune disease in children that can result in severe disability, pain, and loss of quality of life. Similarly to what is found in other autoimmune diseases, the immune-balance in patients with JIA is skewed to a more active and inflammatory phenotype, thereby reacting to “self” and resulting in chronic inflammation in the joints. It is still incompletely understood how immune tolerance is broken in autoimmune diseases, thereby creating a large hurdle to take in the development of novel therapeutic strategies. From a research perspective JIA is a very interesting disease to investigate since it provides a unique opportunity to study the disease directly in the inflamed joints, because the synovium fluid from inflamed joints of these patients is aspirated regularly in our hospital.
The introduction of methotrexate (MTX) and later that of biologicals such as anti-TNFα has led to tremendous progress for the treatment of this disease. However, these treatments are not curative, biologicals are very expensive, have severe side effects, and a sub-population of the patients does not respond to these forms of therapy.
Although treatment with biologicals has proven to be one of the most effective forms of treatment, two main properties of this approach could be improved:
- With biologicals onlya singlepro-inflammatory cytokine is targeted. However, we know that many other disease-driving genes are expressed in inflammatory cells in the joint.
- These biologicals onlyblockcytokines that are already produced, while prevention of expression of disease-driving genes might work better.
We aimed to better understand why the expression of various pro-inflammatory genes is increased in patients with JIA in order to develop novel therapeutic strategies.
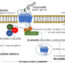
The expression of genes is known to be partly regulated by the “openness” of the DNA. Open stretches of DNA, also known as enhancers, can be used as regulators to slightly modify the expression of nearby genes. Recently the presence of so-called super-enhancers were described. These super-enhancers can influence gene expression on a more profound level.
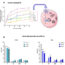
In our recent publication we utilized state-of-the-art techniques to compare the super-enhancer repertoire in healthy controls and JIA patients. We found that super-enhancers that are specifically present in JIA patients correlated with genes that are associated with inflammation and auto-immune diseases, indicating that these super-enhancers are involved in the mechanism of disease. Similarly we demonstrated that these super-enhancers are likely to be involved in other autoimmune diseases such as Rheumatoid Arthritis, Multiple Sclerosis and Lupus. Next we treated cells from JIA patients with a molecule that inhibits super-enhancer activity. In these samples we observed that the expression of more than 600 genes, mostly pro-inflammatory, was increased in JIA patients. Strikingly, the expression of these disease associated genes was preferentially inhibited by this treatment.
Taken together this study was the first to assess the presence of super-enhancers in patients with an immune-related disease. We identified that aberrant super-enhancers can contribute to disease and more importantly that super-enhancer inhibition could be a potential novel therapeutic approach for the treatment of JIA and other autoimmune diseases in the future.
Juvenile Idiopathic Arthritis (JIA) is a leading chronic autoimmune disease in children that can result in severe disability, pain, and loss of quality of life. Similarly to what is found in other autoimmune diseases, the immune-balance in patients with JIA is skewed to a more active and inflammatory phenotype, thereby reacting to “self” and resulting in chronic inflammation in the joints. It is still incompletely understood how immune tolerance is broken in autoimmune diseases, thereby creating a large hurdle to take in the development of novel therapeutic strategies. From a research perspective JIA is a very interesting disease to investigate since it provides a unique opportunity to study the disease directly in the inflamed joints, because the synovium fluid from inflamed joints of these patients is aspirated regularly in our hospital.
The introduction of methotrexate (MTX) and later that of biologicals such as anti-TNFα has led to tremendous progress for the treatment of this disease. However, these treatments are not curative, biologicals are very expensive, have severe side effects, and a sub-population of the patients does not respond to these forms of therapy.
Although treatment with biologicals has proven to be one of the most effective forms of treatment, two main properties of this approach could be improved:
- With biologicals onlya singlepro-inflammatory cytokine is targeted. However, we know that many other disease-driving genes are expressed in inflammatory cells in the joint.
- These biologicals onlyblockcytokines that are already produced, while prevention of expression of disease-driving genes might work better.
We aimed to better understand why the expression of various pro-inflammatory genes is increased in patients with JIA in order to develop novel therapeutic strategies.
The expression of genes is known to be partly regulated by the “openness” of the DNA. Open stretches of DNA, also known as enhancers, can be used as regulators to slightly modify the expression of nearby genes. Recently the presence of so-called super-enhancers were described. These super-enhancers can influence gene expression on a more profound level.
In our recent publication we utilized state-of-the-art techniques to compare the super-enhancer repertoire in healthy controls and JIA patients. We found that super-enhancers that are specifically present in JIA patients correlated with genes that are associated with inflammation and auto-immune diseases, indicating that these super-enhancers are involved in the mechanism of disease. Similarly we demonstrated that these super-enhancers are likely to be involved in other autoimmune diseases such as Rheumatoid Arthritis, Multiple Sclerosis and Lupus. Next we treated cells from JIA patients with a molecule that inhibits super-enhancer activity. In these samples we observed that the expression of more than 600 genes, mostly pro-inflammatory, was increased in JIA patients. Strikingly, the expression of these disease associated genes was preferentially inhibited by this treatment.
Taken together this study was the first to assess the presence of super-enhancers in patients with an immune-related disease. We identified that aberrant super-enhancers can contribute to disease and more importantly that super-enhancer inhibition could be a potential novel therapeutic approach for the treatment of JIA and other autoimmune diseases in the future.
出版
Inhibition of Super-Enhancer Activity in Autoinflammatory Site-Derived T Cells Reduces Disease-Associated Gene Expression.
Peeters JG, Vervoort SJ, Tan SC, Mijnheer G, de Roock S, Vastert SJ, Nieuwenhuis EE, van Wijk F, Prakken BJ, Creyghton MP, Coffer PJ, Mokry M, van Loosdregt J
Cell Rep. 2015 Sep 29












Leave a Reply
You must belogged into post a comment.