Phospholipase A2 group IVc blocks mammary tumor apoptosis
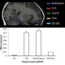
不管无毛鼠(HHR)是一个突变的老鼠rain with autosomal recessive inheritance spontaneously derived from the Sprague-Dawley rat (SDR). The hairless phenotype of HHRs is due to the deletion of basic hair keratin genes, and HHR shows the involution by apoptosis of the mammary gland at an early stage of lactation. Because apoptosis is known to block tumorigenesis in many organs, we hypothesized that HHR may be resistant to mammary tumor development. To examine the hypothesis, the incidence and volume of mammary tumors induced by treatment with 7,12-dimethylbenz[a]anthracene (DMBA) were compared between HHRs and SDRs. The HHR showed the low incidence, volume of mammary tumors were much smaller, and markedly increased apoptotic cells were detected (Fig. 1). Array comparative genomic hybridization and PCR analyses were performed to determine whether additional DNA alterations responsible for cell death are present in HHRs. These examinations revealed the deletion of 50-kb genomic DNA on 1q21, including phospholipase A2 group IVc (Pla2g4c), in HHRs. ThePla2g4cgene was expressed in the ductal carcinoma cells and myoepithelial cells in SDRs but not HHRs.
The Pla2g4c is a member of the cytosolic phospholipase A2family (cPLA2), composed of cPLA2α–ζ. Phospholipase A2(PLA2) is an important enzyme that hydrolyzes membrane phospholipids at thesn-2 position to release unsaturated fatty acids, such as arachidonic acid, which are subsequently metabolized into eicosanoids. Eicosanoids such as prostaglandins and leukotrienes regulate inflammatory responses and cancer pathogenesis.
PLA2s proteins can be divided into families of secretory PLA2s, Ca2+-independent PLA2s, and cytosolic PLA2s (cPLA2). Increased levels of cPLA2α, which is known to play a key regulatory role in carcinogenesis and cell growth, have been reported in human cancers, including those of the bile duct, colon, pancreas, and breast. However, the function of Pla2g4c in cancer development is unknown.

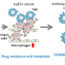
Fig. 2. Pla2g4c blocks mammary tumor apoptosis by inhibiting the nuclear factor-κB/lipocalin 2 pathway.
The direct involvement of Pla2g4c in the prevention of apoptotic cell death was demonstrated through the inhibition of its expression in rat mammary tumor RMT-1 cells by using siRNA. This treatment also induced expression oflipocalin 2(Lcn2) and otherNF-κB-related genes. Pla2g4c siRNA-induced apoptosis was inhibited byLcn2repression or NF-κB inhibitors. NF-κB is often activated in breast cancer to promote cancer cell survival. The transcription factor is also activated in RMT-1 cells by repression ofPla2g4cexpression, but this activation inducesLcn2expression, resulting in cell death.
These results suggest that Pla2g4c seems to function as a repressor against the NF-κB pathway (Fig. 2), although target proteins for blocking remain to be identified. Because PLA2s participate in mammary carcinogenesis, Pla2g4c is also anticipated to become a new possible target for the prevention or treatment of breast cancer.
Publication
Deletion of phospholipase A2 group IVc induces apoptosis in rat mammary tumour cells by the nuclear factor-κB/lipocalin 2 pathway.
Nanashima N, Yamada T, Shimizu T, Tsuchida S
Biochem J. 2015 Jul 15












Leave a Reply
You must belogged into post a comment.