Pd-catalyzed Mizoroki-Heck reaction of [Ph2SR][OTf] with alkenes
Fluoroalkyl(diaryl)sulfonium salts are conventional but useful synthetic reagents in the preparation of functional molecules.S-Trifluoromethyl(diaryl)sulfonium salts, known as the Yagupolskii-Umemoto reagents ([Ar2SCF3]X (X = OTf, BF4)) and initially exploited as electrophilic+CF3transfer sources for a variety of nucleophiles, prove to be effective reagents in the reductive trifluoromethylation of alkenes, alkynes, arenes, andα-diazo esters. 2,2,2-Trifluoroethyl(diphenyl)sulfonium salt, a less well-known reagent which was first synthesized by Umemoto and coworkers, was recently found to be an efficient ylide precursor and trifluoromethylcarbene source for the functionalization of aryl aldehyde, vinyl ketones, and aldimines and olefins, respectively. Compared to the Umemoto and Togni’s reagents, trifluoromethyl- and 2,2,2-trifluoroethyl(diphenyl)sulfonium salts have shown a narrow range of applications. Recently, the Pd-catalyzed arylation of arylboronic acids with [Ar2SCF3][OTf] was reported, which represents the first example of using [Ar2SCF3][OTf] as an arylation reagent.
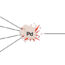
One the other hand, the Pd-catalyzed arylation of alkenes has been extensively investigated by a large number of research groups around the world using aryl halides or tosylates, azidoarenes, and diaryliodinium salts as electrophiles. In this work, we disclosed the Pd-catalyzed Mizoroki-Heck phenylation of alkenes with phenylsulfonium salts including the Yagupolskii-Umemoto reagents. To our delight, a variety of alkenes were efficiently phenylated by [Ph2SCF3][OTf] (2a) or [Ph2SCH2CF3][OTf] (2b) in the presence of 10 mol% Pd[P(t-Bu)3]2under mild and acidic reaction conditions. Styrenes bearing either electron-donating or -withdrawing groups on the phenyl rings were smoothly converted, which gave the corresponding phenylation products in good to high yields. The unconjugated olefin, enolate, and electron-deficient alkenes were also suitable substrates in the conversion (Fig. 1B).In addition, phenylsulfonium salts (2c-j) bearing either alkyl or fluoroalkyl groups on the sulfur atoms were available phenylation reagents in the Pd-catalyzed Mizoroki-Heck cross-coupling (Fig. 1C). 1-Methoxy-4-vinylbenzene (1a) reacted with the commonly used phenylation reagents, such as benzenediazonium tetrafluoroborate (2k), iodobenzene (2m), phenyl triflate (2n), and tetraphenylphosphonium bromide (2o), under the standard conditions to give much lower yields of 3a, while the reaction of 1a with diphenyliodonium triflate (2l) provided a yield comparable to that from 2b. These results indicated that phenylsulfonium triflates (e.g., 2a, 2b, 2d, 2e, 2g, and 2h) are the powerful cross-coupling participants among all the tested reagents in Pd-catalyzed Mizoroki-Heck reaction with alkenes at room temperature (Fig. 1D).
Overall, the present reaction provides an efficient and facile access to aromatic olefins under mild conditions, which represents the first use of alkylphenylsulfonium salts as phenylation reagents in the Pd-catalyzed Mizoroki-Heck reaction.
Hai-Xia Song, Shi-Meng Wang, Xiao-Yan Wang, Cheng-Pan Zhang
School of Chemistry, Chemical Engineering and Life Science, Wuhan University of Technology, Wuhan, China
Publications
Palladium-catalyzed Mizoroki-Heck-type reactions of [Ph2SRfn][OTf] with alkenes at room temperature.
Wang SM, Song HX, Wang XY, Liu N, Qin HL, Zhang CP
Chem Commun (Camb). 2016 Sep 29











Leave a Reply
You must belogged into post a comment.