Chemoselective Intramolecular carbene C-H insertions of 2-diazo-2-sulfamoylacetamides
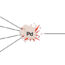
The transition-metal-catalyzed intramolecularly aliphatic and aromatic C-H insertions of diazo amides provide useful and important transformations to efficiently synthesize nitrogen-containing heterocycles with the new generated C-C bond. The reactivity of different diazoamide derivatives has been well studied by us and others (Scheme 1a), affording various products, including β-lactams (I), γ-lactams (II), indolin-2-ones (III), 1,4-dihydroisoquinolin-3(2H)-ones (IV), Buchner products (V), and cyclopropanation products (VI) (Scheme 1a) and a series of exquisite reactions has been established to construct complex and useful structures, such as biological and natural products.

Fig. 1. Intramolecular carbene C-H insertions of diazoacetamide derivatives and acyl diazomethanesulfonamides.
The chemoselective intramolecular C-H insertions of carbenes derived from 2-diazo-2-sulfamoylacetamides were studied. 2-Diazo-2-sulfamoylacetamides were first prepared from chloroacetyl chloride and secondary amines through acylation followed by sequential treatments with sodium sulfite, phosphorus oxychloride, secondary amines, and 4-nitrobenzenesulfonyl azide. The results of their Cu(acac)2-catalyzed intramolecular carbene C-H insertions indicate that: (1) 2-diazo-N,N-dimethyl-2-(N,N-diphenylsulfamoyl)acetamide undergoes the formal aromatic 1,5-C-H insertion in itsN-phenylsulfonamide moiety to afford the corresponding 1,3-dihydrobenzo[c]isothiazole-3-carboxamide 2,2-dioxide derivative; (2) no aliphatic C-H insertions occur for 2-diazo-2-(N,N-dialkylsulfamoyl)acetamides; and (3) for 2-diazo-N-phenyl-2-(N-phenylsulfamoyl)acetamides, the formal aromatic 1,5-C-H insertion in theN-phenylacetamide moiety is favorable to afford the corresponding 3-sulfamoylindolin-2-one derivatives as sole or major products. The intramolecular competitive aromatic 1,5-C-H insertion reactions of 2-diazo-2-sulfamoylacetamides with aryl groups on both amide and sulfonamide groups reveal that theN-aryl substituents on acetamide are more active than those on sulfonamide. The chemoselectivity is controlled by electronic effect of the aryl group.
Chuqiang Que, Peipei Huang, Zhanhui Yang, Chen, Ning Chen, Jiaxi Xu
State Key Laboratory of Chemical Resource Engineering, Department of Organic Chemistry,
College of Chemistry, Beijing University of Chemical Technology, Beijing, China
Publication
Intramolecular Carbene C-H Insertion Reactions of 2-Diazo-2-sulfamoylacetamides
Chuqiang Que, Peipei Huang, Zhanhui Yang, Ning Chen, Jiaxi Xu
Molecules. 2019 Jul 19









Leave a Reply
You must belogged into post a comment.