p53 gene reactivation reverts hypoxic resistance in KRAS mutant tumor cells decreasing glucose uptake
When growing distant from primary blood supply sites, tumor cells face hypoxia characterized by niches restricted in oxygen and glucose. Cells acquiring resistant to hypoxic stress undergo metabolic re – programming, and increased resistance to 3 – BrPA,(3-bromopyruvate), a pro-oxidant anti-tumor agent capable of inhibiting glycolytic and mitochondrial targets and increasing free radicals. 3-BrPA at 110 μM was shown to suppress the growth of colorectal carcinoma cells with KRAS or BRAF mutations surviving transient aerobic glucose starvation. In contrast, we showed in aerobic ERα positive breast cancer cells, that a functional wt p53 conferred resistance to 3-BrPA, since p53 silencing, or use of genetically matched cells with mutant p53 R175H, revealed high susceptibility to 75 μM 3-BrPA. The wt p53-induced resistance to 3-BrPA was independently confirmed in RT4 (grade I; wild-typep53) bladder cancer cells that remained unaffected by 125 μM 3-BrPA, in contrast to T24 (grade III; mutantp53) bladder cancer cells, which greatly diminished their survival at comparable 3-BrPA concentrations.

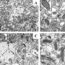
Fig. 1. A) NAC counteracts toxicity of Prima-1 and 3-BrPA in hypoxic (G12S)-mutant KRAS-A549 cells
Crystal violet staining of surviving cells was used to compare the response to a 72 hour treatment with Prima-1 or 3-BrPA in A549 cultures under hypoxia in complete Dulbecco’s medium containing 20 mM glucose, 4 mM glutamine supplemented with 10% serum. B&C). Prima-1 and 3-BrPA cooperate to inhibit cell cycle progression and promote hypoxic cell death is antagonized by NAC Cell cycle analysis and assay of below_2n dead cells was performed as indicated under Methods for cells cultured under hypoxia for 48 hours in 20mM glucose, 4 mM glutamine supplemented with 10% serum, or 5 mM glucose, 2 mM glutamine and 5 % dialyzed serum.
* denotes significance between treated cells relative to controls.
Wild type p53 tumor suppressive functions may be inactivated by critical effectors of oncogenic KRAS signaling like Snail, Notch1 or Ral GTPases. In this report ,we show resistance to 3-BrPAonly根据缺氧(≤2%的氧气)在人类肺癌A549亚丁湾ocarcinoma cell line harbouring a wt p53 gene and a KRAS gene mutation (G12S) and in C8161 cells having a wt p53 gene with an enhancing KRAS G12D mutation and a G464E mutation in the BRAF P loop region. In contrast, hypoxic mutant NRAS MelJuso cells are highly susceptibile to 3-BrPA or Prima-1. Since metabolic stress is particularly increased by hypoxia in KRAS-mutant cancer cells leading to overexpression of GLUT1 glucose receptor in resistant cells to increase glucose transport, aiming to compensate for the resulting glucose starvation, we hypothesized that GLUT-1 expression could be antagonized by PRIMA-1, reported by our laboratory to reactivate wt p53 in hypoxic estrogen receptor α+,wt p53 breast cancer cells and hypoxic mutant p53 Her-2 mutant breast cancer cells.
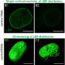
Reverse transcription-PCR and immune fluorescence showed that Prima-1 decreases GLUT-1 receptor expression in KRAS-mutant cells. Besides its role as a p53 reactivator in both wt p53 or mutated p53 cells, Prima-1 possibly counteracts hypoxic resistance to 3-BrPA in some KRAS-mutant wt p53 cancer cells by potentiating 3-BrPA metabolic damage and increasing oxidative stress, since the anti-oxidant N-acetylcysteine was able to reverse the toxicity jointly induced by Prima-1 and 3-BrPA.
SIGNIFICANCE.- , this report is the first showing that Prima-1 overcomes the resistance to 3-BrPA in hypoxic wt p53 KRAS-mutant cells by promoting wt p53 reactivation, inhibition of glucose uptake by tumor cells and pro-oxidant cancer therapeutics, is important given that survival under hypoxia contributes to tumor progression and because no effective single clinical therapy has been consistently achieved to treat tumors linked to KRAS
Highlights
- Hypoxia increases resistance to 3-bromopyruvate (3-BrPA) in KRAS-mutant wt p53 cells
- Prima-1, a p53 reactivator decreases GLUT1 and counteracts hypoxic resistance to 3-BrPA
- N-acetylcysteine reverts toxicity induced by Prima-1 and 3-BrPA
Andrea Orue, Valery Chavez, Mary Strasberg-Rieber, Manuel Rieber
IVIC, Tumor Cell Biology Laboratory, Apartado 21827, Caracas, Venezuela
Publication
Hypoxic resistance of KRAS mutant tumor cells to 3-Bromopyruvate is counteracted by Prima-1 and reversed by N-acetylcysteine.
Orue A, Chavez V, Strasberg-Rieber M, Rieber M
BMC Cancer. 2016 Nov 18








Leave a Reply
You must belogged into post a comment.