Lipid nanoparticles loaded with CpG oligonucleotides exert an antitumor effect via immunomodulation
Since the emergence of immune checkpoint inhibitors (ICIs), cancer immunotherapy has dramatically developed. However, its drawback is the low overall response rate to its therapeutic effect. The efficacy of immunotherapy is suggested to depend on the immune status of the tumor microenvironment (TME). In general, a tumor with low infiltrating lymphocytes (cold tumor) is resistant to immunotherapy with ICIs. Thus, therapeutic strategies could convert TME from cold tumor to immune-activating TME (hot tumor), which stimulates tumor-infiltrating lymphocytes to promote antitumor immune response.
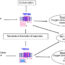
Type-A CpG oligodeoxynucleotide (ODN), D35, has the potential to induce interferon-α (IFN-α) by activating toll-like receptor 9 (TLR9) of plasmacytoid dendritic cells. IFN-α is known as an immunomodulator to augment antitumor efficacy. Thus, D35 is expected to induce an effective antitumor immune response. However, D35 is very difficult to handle due to the formation of uncontrolled large aggregates in salt-containing buffers. Furthermore, D35 has a poor cellular uptake due to electrostatic repulsion between the cell membrane and negatively charged ODNs. To overcome these problems, we developed D35-loaded lipid nanoparticles (D35LNP) and evaluated the antitumor effect using murine colon carcinoma-inoculated model.
First, we designed D35LNP consisting of four lipids, namely, cationic lipid, phospholipid, cholesterol, and polyethylene glycol-modified lipid (PEGylated lipid). D35LNP is prepared by mixing lipid solution and D35 aqueous solution with a microfluidic system. D35LNP highly induced IFN-α via specific recognition of TLR9 from human peripheral blood mononuclear cells. We also evaluated the effect of PEGylated lipids on IFN-α induction. IFN-α induction was decreased depending on the amount of the PEGylated lipid. This result indicated that the steric hindrance of PEG chains prevented D35LNP uptake into the cells. Because PEG-modified nanoparticles prevent phagocytosis by the reticuloendothelial system and prolong the time of blood circulation, we examined the effect of the different PEG modifications in D35LNP (low: D35LNP0.5%, high: D35LNP3.0%) on the antitumor effect for the murine colon carcinoma (MC-38)-inoculated model. Then, tumor growth was significantly suppressed by D35LNP0.5%, but not D35LNP3.0%, administered via intratumoral injection. In addition, the antitumor effect of D35LNP0.5% was completely canceled in CD8+T cell-depleted mice. These results indicated that D35LNP0.5% induced antitumor effect by activating CD8+T cells. Moreover, the gene expression levels of IFNs were significantly increased in the tumor tissue by D35LNP0.5% treatment. Based on these results, D35LNP0.5% was thought to convert the TME to be more sensitive for immunotherapy.
The antitumor effect was also evaluated via intravenous injection. D35LNP0.5%, but not D35LNP3.0%, suppressed tumor growth. In addition, it was confirmed that the expression levels of antitumor genes, such as IFN-γ, CXCL9, and STAT4, in the tumor tissue were significantly increased by injection with D35LNP0.5% compared with D35LNP3.0%. Thus, the cellular uptake of D35LNP was expected to induce the expressions of antitumor genes and exert antitumor effects. The antitumor effect exerted by the combination therapy of D35LNP0.5% and anti-PD-1 antibody (ICI) was also examined. Although the combination therapy did not significantly increase the antitumor effect (because the anti-PD-1 antibody itself strongly suppressed tumor growth), it increased CD8+T cells and decreased immunosuppressive cells in the tumor tissue.
In conclusion, we optimized D35LNP as a novel antitumor agent used to overcome the drug formulary issues of Type-A CpG ODNs. Furthermore, it was highlighted that D35LNP exerts an antitumor effect by activating CD8+T cells. The combination therapy of anti-PD-1 antibody and D35LNP enhanced the antitumor immune response. Therefore, D35LNP is considered a novel antitumor immunostimulatory agent capable of converting TME.
Lisa Munakata1,Taiki Aoshi2,Ryo Suzuki1,3
1Laboratory of Drug and Gene Delivery Research, Faculty of Pharma-science, Teikyo University, Japan
2Department of Cellular Immunology, Research Institute for Microbial Diseases, Osaka University, Japan
3Advanced Comprehensive Research Organization (ACRO), Teikyo University, Japan
Publication
Lipid nanoparticles of Type-A CpG D35 suppress tumor growth by changing tumor immune-microenvironment and activate CD8 T cells in mice
Munakata L, Tanimoto Y, Osa A, Meng J, Haseda Y, Naito Y, Machiyama H, Kumanogoh A, Omata D, Maruyama K, Yoshioka Y, Okada Y, Koyama S, Suzuki R, Aoshi T
J Control Release. 2019 Nov 10













Leave a Reply
You must belogged into post a comment.