Is VPS35 critical to prevent Parkinson’s disease?
Parkinson’s Disease (PD) is a severely debilitating motor disorder characterized by loss of dopaminergic neurons and increase of α-synuclein in a brain region known as the substantia nigra pars compacta (SNpc). Although much progress has been made in uncovering potential mechanisms underlying PD pathogenesis, identification of how implicated mechanisms may lead to PD pathology in the central nervous system (CNS) has remained elusive. Investigation of genes associated with PD has been of great assistance in these endeavors, guiding researchers down various paths of investigations in hopes of uncovering how dysfunction of individual molecular pathways could ultimately result in PD neuropathology.
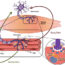
One such gene which has been implicated in PD is a gene calledVPS35. The protein encoded by this gene is an essential subunit of an intracellular protein complex known as the retromer. Retromer dysfunction is known to cause abnormal functioning of multiple molecular pathways important in CNS function. In our studies, we sought to determine through which molecular pathways VPS35 and retromer dysfunction might contribute to PD pathology. In individually published manuscripts, we reported on two independent pathways through which VPS35 deficiency and VPS35 mutation work to affect neurodegenerative pathology in the SNpc. One of the pathways altered by retromer dysfunction impaired the function & performance of mitochondria – intracellular organelles essential for cellular respiration and energy production. By restoring this particular pathway, we were able to recover the loss of dopaminergic neurons in our model, but not the accumulation of α-synuclein. We found that the α-synuclein accumulation in our model was affected by impaired function of a lysosomal pathway through which α-synuclein is removed from neurons by a process called autophagy – a cellular degradation system important for the removal of cytoplasmic debris. By restoring this pathway, we were able to ameliorate the α-synuclein accumulation.
Not only do our studies show a direct cause-and-effect relationship between VPS35 and PD neuropathology, but they show that this particular protein is essential for intracellular mitochondrial and lysosomal function and that both deficiency of VPS35 and mutation of theVPS35gene impair these vital components of cellular function, ultimately resulting in neurodegenerative pathology. These studies have revealed important mechanisms underlying PD pathogenesis, which is imperative for future development of therapeutic & preventative measures.
Joanna Erion, Fulei TangandWen-Cheng Xiong
Department of Neuroscience and Regenerative Medicine, Medical College of Georgia, Augusta, GA, USA
Publication
VPS35 Deficiency or Mutation Causes Dopaminergic Neuronal Loss by Impairing Mitochondrial Fusion and Function.
Tang FL, Liu W, Hu JX, Erion JR, Ye J, Mei L, Xiong WC
Cell Rep. 2015 Sep 8











Leave a Reply
You must belogged into post a comment.