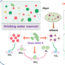
Washed platelet concentrates prevent recurrent transfusion reactions
Platelet transfusion is commonly used to prevent or treat bleeding in people with thrombocytopenia or platelet dysfunction. Among transfusable blood products, however, platelet concentrates (PCs), which also contain plasma, have a higher frequency of transfusion reactions than red blood cells or fresh frozen plasma. Transfusion reactions can lead to discomfort for the patient and can even be fatal, albeit rarely. Management of these is therefore crucial in terms of continuity of transfusion and quality of life. Plasma is considered to be candidates for the cause of transfusion reactions and removal of plasma in PCs by washing (Fig. 1) effectively reduces transfusion reactions by some clinical studies. In Japan, washed PCs (WPCs) are approved as blood products and have been provided by the Japan Red Cross Society (JRCS) since 2016.
We conducted a national survey to evaluate the efficacy and safety of WPC supplied by the JRCS blood center. In the manufacturing of WPCs, leukoreduced apheresis PCs were washed with bicarbonate Ringer’s solution A and automated closed-system cell processor. In 50 institutes, which used WPCs between September 2016 and January 2017, 91 patients and 1,210 bags of 10 units each of WPCs were evaluated. This covered about 30% of WPCs by JRCS during the study period. Patients displayed mostly hematological diseases and had a median of 48 transfusion histories. The median number of WPCs per patient was 8 (range, 1–91). WPCs were transfused to 86 patients (94.5%) due to recurrent PC-related transfusion reactions that could not be prevented by premedication with antihistamine and hydrocortisone.
女警官临床预防效果的实现d in 99.8% of the transfusions, and the recurrent transfusion reactions by WPCs were confirmed in only 2 of 1,206 transfusions. Subgroup analysis revealed that the preventative effect was not affected by sex, disease, type, or severity of transfusion reactions. WPCs were therefore safely transfused into patients with a history of severe transfusion reactions such as anaphylaxis and respiratory distress. Adverse reactions associated with WPC were observed in 9 (0.7%) patients and included pruritus (n = 5), urticaria (n = 2), chills/rigor (n = 1), and chest discomfort (n = 1). These were equivalent in incidence rate to those of previous studies for WPCs made at medical institutes. Bacterial growth due to reduction of complement components in the plasma were concerning, but bacterial infections due to WPCs were not observed.
As a disadvantage of WPCs, about 10%–20% of platelet loss was reported in the washing process, and this may be the cause of platelet transfusion refractoriness. Platelet increase 24 hours after WPC transfusion was observed at a median of 3.5 (range, −13 to 53.6) × 109/L, lower than those reported in previous studies. However, platelet values at 24 hours could be evaluated in only 36 patients, and 11 patients had risk factors for platelet transfusion refractoriness such as bleeding and infections. The median pretransfusion platelet count was 18 (range, 0–130) × 109/L; this was considered to be a high value compared with the transfusion threshold of clinical guidelines. This may be because WPCs are used in routine practice and cancellation of WPCs was not allowed.
In conclusion, this retrospective multicenter study was the first to evaluate the clinical effects of commercially available WPCs. WPCs were effective for recurrent transfusion reactions in almost all transfusions. Further studies are needed to evaluate both transfusion efficacy and healthcare cost of WPCs, and preparation of guidelines for WPC indications and contraindications is desirable.
Shin-ichiro Fujiwara
Division of Cell Transplantation and Transfusion, Jichi Medical University Hospital, Japan
Publication
Released Washed Platelet Concentrates Are Effective and Safe in Patients With a History of Transfusion Reactions
Fujiwara SI, Fujishima N, Kanamori H, Ito M, Sugimoto T, Saito S, Sakaguchi T, Nagai K, Masuoka H, Nagai K, Morita A, Kino S, Tanaka A, Hasegawa Y, Yokohama A, Fujino K, Makino S, Matsumoto M, Takeshita A, Muroi K
Transfus Apher Sci. 2018 Dec













Leave a Reply
You must belogged into post a comment.