Smart tumor suppression via intracellularly delivered drug
With the rapid metabolism of tumor cells, the microenvironments of tumor tissue and cells present acidity, while the pH maintains at about 7.4 in normal extracellular matrices and blood. In detail, the extracellular pH in tumor tissue is about 6.8 and the intracellular endo/lysosomal pH is about 5.5. The different pH values of different components provide a breakthrough for delivering drug into the tumor regions using a pH-responsive polymer-based drug delivery platform.

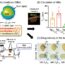
Fig. 1. Schematic illustration of preparation of pH-responsive PIC micelle, and its circulation in vivo, accumulation in tumor tissue, and final pH-triggered intracellular DOX release after intravenous injection.
Polymeric micelles formed from amphiphilic copolymers in aqueous solutions have been extensively explored as nanoscale carriers for hydrophobic antitumor drugs, which can prolong the circulation time by avoiding rapid clearance by the renal and reticuloendothelial systems. Therefore, the pH-responsive polymeric micelles have been widely developed to release drugs with efficacy into tumor tissues.
Herein, a pH-responsive polyion complex (PIC) micelle from anionic acid-sensitive methoxy poly(ethylene glycol)-block-poly(N(ε)-((1-carboxy-cis-cyclohexene)-2-carbonyl)-L-lysine) (mPEG-b-PCLL) and cationic doxorubicin (DOX), a model anthracycline antitumor drug, is constructed (Fig. 1). The PIC micelle exhibits varying diameters and DOX release rates at different pH values. Apparently, we can foresee that the DOX will release only when the PIC micelle reaches into the tumor cells in the organism. This process can not only avoid the toxicity of premature release of DOX in the body but also improve the drug concentration in tumor location.

Fig. 2. Evaluations of tumor volume (A), body weight (B), tumor inhibition rate (C), and tumor weight (D) of H22-xenografted BALB/c mice after treatments with free DOX and PIC micelle employing NS as a control. The data are represented as mean ± standart deviation (n = 8; #p < 0.001).
And then, we test the tumor suppression efficiency of PIC micelle on mouse hepatoma H22-xenografted tumor model compared with free DOX as a human body simulation experiment. The PIC micelle can much effectively suppress the growth of tumor and avoid the weight loss of mice (Fig. 2). In addition, the further histopathological and immunohistochemical analyses also prove a more excellent antitumor performance of PIC micelle compared to free DOX.
In summary, with convenient fabrication, controlled intracellular release, and satisfactory antitumor efficiency, the pH-responsive drug-incorporated PIC micelle may become a potential platform for clinical malignancy chemotherapy.
Publication
Selective intracellular drug delivery from pH-responsive polyion complex micelle for enhanced malignancy suppression in vivo.
Wang J, Xu W, Guo H, Ding J, Chen J, Guan J, Wang C
Colloids Surf B Biointerfaces. 2015 Jul 26










Leave a Reply
You must belogged into post a comment.