Placental transfer of the HIV drug dolutegravir in a human ex vivo perfusion model
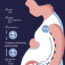
The fetus of an HIV-positive woman has a 25% chance of becoming infected during pregnancy. HIV medicines have shown to be highly effective in preventing fetal infection with HIV. A part of the efficacy but also the safety of an HIV drug will depend on the amount that is able to reach the fetus during pregnancy. Fetal drug exposure depends largely on the capacity of a drug to transfer across the placenta. However, for most new anti-HIV drugs placental transfer unknown. Investigators from the Departments of Pharmacy, Pharmacology and Toxicology, and Obstetrics and Gynecology of Radboud University Medical Center (Nijmegen, The Netherlands) developed a placenta perfusion model to estimate the maternal to fetal transfer of the new HIV drug, dolutegravir.
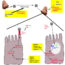
已经使用previousl胎盘灌注模型y to successfully predict the transfer of anti-HIV drugs across the placenta. For these models, placentas of healthy women are collected directly after delivery. A functional placental unit, a cotyledon, is selected and mounted into a perfusion apparatus, where it can be kept viable for about 4 hours. The system mimics the conditions of the end stage of a normal pregnancy, including a fetal and maternal blood circulation, which are separated by the cotyledon.
In this study dolutegravir was administered to the maternal circulation. The amount of dolutegravir that was added reflected the blood concentration of an HIV-positive pregnant woman using dolutegravir. Over a period of three hours a concentration of dolutegravir in the fetal circulation was reached, which was about 60% of the maternal concentration.
This indicates that dolutegravir transfer across the placenta is high and fetal drug exposure most likely substantial as compared to other HIV medicines. Based on these results, dolutegravir bears potential for the prevention of fetal HIV infection during pregnancy. However, fetal drug exposure to dolutegravir also implies a risk of fetal toxicity. Overall, clinical studies need to confirm these findings and provide reliable data on the efficacy and safety of dolutegravir use in pregnancy.
Publication
Placental transfer of the HIV integrase inhibitor dolutegravir in an ex vivo human cotyledon perfusion model.
Schalkwijk S, Greupink R, Colbers AP, Wouterse AC, Verweij VG, van Drongelen J, Teulen M, van den Oetelaar D, Burger DM, Russel FG.
J Antimicrob Chemother. 2016 Feb










Leave a Reply
You must belogged into post a comment.