Nuclear membrane diversity
Although it is obvious that every tissue in our bodies is different, the vast majority of scientific research is done on a small number of cancer cell lines studying individual cells grown in dishes — for example the famous Henrietta Lacks HeLa cell line. This approach was embraced because it was thought that most cellular processes are relatively uniform and a liver cell in a dish does not look very different from a kidney or lung cell in a dish. Moreover, the gross structural features of organelles within cells appear similar, for example mitochondria, vacuoles, endosomes, endoplasmic reticulum, Golgi apparatus, and the nuclear envelope that forms the barrier separating the genetic material from the rest of the cell.
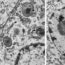
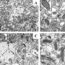
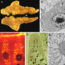
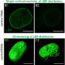
However, several recent studies reviewed in the article by Worman and Schirmer using the method for high-throughput protein detection known as “proteomics” have demonstrated that, although it looks similar, the proteins embedded the nuclear envelope in each cell differs enormously between different tissues. In fact, only about 15% of these nuclear membrane proteins were shared between different tissues. Several other recent studies have shown that these tissue-specific nuclear membrane proteins play important roles in the regulation of cell division, the organization of proteins that give cells in individual tissues their particular shape, and tissue-specific patterns of genome organization that contribute to gene expression. For example, the nuclear membrane connects to proteins in the cytoplasm that help neurons to send signals in a particular direction (Fig. 1 polarized signaling) and in blood cells to proteins that direct how immune cells get activated to target pathogens (Fig. 1 antigen presentation). In muscle nuclear membrane proteins are important for accumulating the multiple nuclei at the neuromuscular junction so that signals from nerves innervating the muscle can be rapidly interpreted for gene expression responses (Fig. 1 nuclear migration). Some tissues have less need for nuclear connections in this direction (Fig. 1 fat), but nonetheless have important functions in the other direction where membrane proteins on the inside of the nuclear membrane connect to and regulate the genome. Most importantly, some of these tissue-specific nuclear membrane proteins are critically important for tissue differentiation and many recent studies are finding that they are mutated in a variety of human diseases where pathology is focused in particular tissues. Thus far the proteins in the nuclear membrane have only been analyzed in a handful of tissues, but bioinformatics analysis suggests that there are many more tissue-specific nuclear membrane proteins to be discovered when other cell types are similarly analyzed by proteomics. That functions have only been identified thus far for a small handful of these tissue-specific nuclear membrane proteins and that these functions are so important, suggests that the nuclear membrane “proteome” may become a scientific Rosetta Stone that will unlock many mysteries of tissue-specific cell functions and disease pathologies.
Publication
Nuclear membrane diversity: underlying tissue-specific pathologies in disease?
Worman HJ, Schirmer EC.
Curr Opin Cell Biol. 2015 Jun









Leave a Reply
You must belogged into post a comment.