Improvement of critically sized bone fracture healing by using conductive tissue engineering scaffolds
Bone is the second most prevalent transplant tissue after blood. Healing the critically sized bone defects caused by tumor or trauma are among the major challenges for orthopedic surgeons.
Although, allografts and autografts are commonly used for treatment of large bone defects, there are some concerns for these types of treatment such as immunocompatibility issues, inadequate availability of transplantable bone, and donor site morbidity. Therefore, three dimensional (3D) porous constructs known as the tissue engineering scaffolds with structure similar to the natural bone are being developed using synthetic and natural biomaterials.

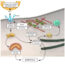
Fig. 1. Scanning electron microscopy image from adult human mesenchymal stem cells cultured on the produced conductive scaffolds showing the appropriate cell/scaffold interactions.
While efforts in the scaffold design for bone tissue engineering are mostly focused on improving the biocompatibility, bioactivity, and biodegradability of 3D porous scaffolds, in our research we tried to improve the electromagnetic properties of such scaffolds. Improving the electromagnetic characteristic of tissue engineering scaffolds, which is usually considered in nerve tissue engineering, is known as a key factor to enhance the signaling among growing cells. Moreover, considering the improvement of bone healing by applying the electromagnetic stimulation, development of conductive bone scaffold is on demand to facilitate the local delivery of electromagnetic stimulation to the injured region.
Herein, we adjusted the conductivity of bioactive-glass bone scaffolds by addition of poly(3,4-ethylenedioxythiophene):poly(4-styrene sulfonate) (PEDOT:PSS) as a biocompatible conductive polymer. Concentration of PEDOT:PSS inside the scaffolds was optimized to design the most appropriate scaffold in terms of biocompatibility and bioactivity. We have shown that an optimized bioactive glass conductive scaffold can improve the cell viability more than 4 times compared to an equivalent nonconductive scaffold. Note that, increasing the conductivity more than the optimum point may have reverse effect on the cell proliferation.
In addition, immersing the scaffolds in simulated body fluid and characterization by scanning electron microscopy, energy dispersive spectroscopy, X-ray diffraction analysis and Fourier transform infrared spectroscopy confirmed the improved biomineralization activity of the produced conductive scaffolds. The results of bioactivity experiments revealed the formation of bone-like apatite deposited on the surface of the scaffold, which can confirm the increased ability of scaffold integration to the surrounding host tissue.
This class of scaffold may have significant influence on the future of bone treatments, especially for critically sized bone defects in sensitive body areas which require rapid healing.
Publication
Biomineralization and biocompatibility studies of bone conductive scaffolds containing poly(3,4-ethylenedioxythiophene):poly(4-styrene sulfonate) (PEDOT:PSS).
Yazdimamaghani米、哈米,Mozafari M, Vashaee D, Kotturi H, Tayebi L.
J Mater Sci Mater Med. 2015 Dec











Leave a Reply
You must belogged into post a comment.