GSK-3 beta: a therapeutic target for Parkinson’s disease
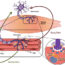
Neurogenesis is a process of birth of newborn neurons from neural stem cells (NSCs). It is a complex, multistep process which involves NSC proliferation, differentiation, migration, maturation and integration of newborn neurons into existing neuronal circuitry. Continuous neurogenesis requires a fine-tuned balance between NSC proliferation and differentiation. Insufficient formation or accelerated degeneration of newborn neurons may contribute to motor/nonmotor symptoms of Parkinson’s disease (PD). Degeneration of dopaminergic (DAergic) neurons in substantia nigra (a brain region rich in dopaminergic neurons) is a characteristic feature of PD that results in development of motor and hippocampal (brain part important for memory functions) associated nonmotor symptoms. Reduced adult hippocampal neurogenesis is associated with deficits in hippocampal dependent behavioural functions including impairment in learning and memory.

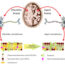
This raises the possibility that nonmotor symptoms in PD could be a result of reduced hippocampal neurogenesis. Several developmentally conserved signaling pathways including Wnt and Notch are associated with modulation of adult neurogenesis during physiological and pathological state. Glycogen synthase kinase-3β (GSK-3β) is a key modulator of Wnt/β-catenin and Notch signaling pathways which are crucially important for NSC proliferation and subsequent lineage commitment during differentiation. Therefore dysregulation of GSK-3β is linked with several neurological disorders, including Schizophrenia, Alzheimer’s disease and PD. However, several questions related to neurogenesis and PD pathogenesis remains to be addressed, including 1) whether GSK-3β in subventricular zone (SVZ) and dentate gyrus of hippocampus, two key areas where neurogenesis has been found to occur in the adult mammalian brain, is modulated by intra-medial forebrain bundle (MFB, a neural pathway important for reward system in brain) injection of a neurotoxin 6-hydroxydopamine (6-OHDA); 2) how this affects multiple steps of adult neurogenesis in rat model of PD-like phenotypes.
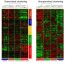
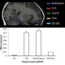
To specifically investigate our questions, we employed 6-OHDA induced rat model of PD-like phenotypes that we established for investigating the role of GSK-3β in adult neurogenesis in PD pathogenesis. Single unilateral intracranial injection of 6-OHDA into MFB resulted in early and persistent activation of GSK-3β in SVZ and hippocampus. The neural progenitor cells (NPCs) in 6-OHDA-lesioned rats divide much faster than age matched control rats as indicated by increased labeling index in SVZ and hippocampus. Interestingly, 6-OHDA lesioned PD rats exhibited reduced NSC proliferation, neuroblasts migration, dendritic arborization, neuronal differentiation and enhanced glial (non neuronal cells of nervous system) differentiation in SVZ and hippocampus region. The aforementioned effects were involved in the downregulation of Wnt/b-catenin signaling and upregulation of Notch signaling in PD rats.
To investigate the role of GSK-3β in regulating NSC proliferation and fate specification in rat model of PD-like phenotypes, we employed pharmacological approach by systemically injecting SB216763, a specific GSK-3β inhibitor. Pharmacological inhibition of GSK-3β potentially enhanced Wnt/β-catenin signaling and reduced Notch signaling pathway associated proteins in SVZ and hippocampus of PD rats. GSK-3β inhibition reduced NPC division rate and increased reentry index that might have resulted in enhanced NSC proliferation and long-term survival in PD rats, thus suggesting a role of GSK-3β in control of cell cycle. Interestingly, GSK-3β inhibition significantly enhanced expression of doublecortin+(DCX+), a marker of immature neurons and stimulated their migration towards rostral migratory stream (path through which NSC migrate) and lesioned striatum (brain part important for locomotor functions) from SVZ in PD rats. The dendritic length and arborization of immature neurons was also recovered following GSK-3β inhibition in PD rats, indicating that GSK-3β inhibition promotes survival of immature neurons against neurotoxic insults. Notably, GSK-3β inhibition enhanced number of mature newborn neuron in hippocampus and reduced formation of mature astrocytes in SVZ and hippocampus in PD rats, suggesting that GSK-3β inhibition shifts the NSC fate specification from glial differentiation to neuronal differentiation. Therefore, increased NSC pool and their neuronal differentiation lead to enhanced survival of immature neurons that resulted in improved net adult neurogenesis in PD rats which requires fine-tuned balance between Wnt and Notch signaling. Notably, any treatment designed to enhance neurogenesis or reduce DAergic degeneration could be effective in reversing the early PD pathology.
Sonu Singh, Akanksha Mishra, Shubha Shukla
PCS-203, Department of Pharmacology, CSIR-Central Drug Research Institute (CSIR-CDRI), Sector 10, Jankipuram extension, Sitapur Road, Lucknow (UP)- 226031, India
Publication
Glycogen Synthase Kinase-3β Regulates Equilibrium Between Neurogenesis and Gliogenesis in Rat Model of Parkinson’s Disease: a Crosstalk with Wnt and Notch Signaling.Singh S, Mishra A, Bharti S, Tiwari V, Singh J, Parul, Shukla S
Mol Neurobiol. 2018 Aug









Leave a Reply
You must belogged into post a comment.