An international team opens new avenues toward PKU therapy
When a baby is born, a simple blood test reveals whether the child has PKU, which is a recessive genetic disorder where the body fails to regulate the amount of phenylalanine (Phe) in blood. Phe is a required nutrient found in foods containing protein. High Phe levels during infancy and childhood cause severe and irreversible neurological damage. A positive diagnosis occurs in approximately 1 out of 12,000 infants. Since the mid-20thCentury, neonatal testing and early dietary intervention has prevented the severe consequences of high Phe during brain development. Uncontrolled Phe levels in adolescents and adults can result in lapses in rational behavior and a need for psychiatric care. Thus, 21stCentury guidelines recommend that PKU-affected individuals control blood Phe levelsthroughout life. Since maintaining a protein-restricted diet is problematic, individuals living with PKU often struggle to maintain safe Phe levels. Required new therapies can emerge from understanding the enzyme phenylalanine hydroxylase (PAH) whose dysfunction results in PKU. Research published in美国国家科学院院刊》上s USA,on February 16, 2016, provides important new insight into how PAH works to control blood Phe. The published work was carried out by an international team led by Eileen K. Jaffe of the Fox Chase Cancer Center – Temple Health.

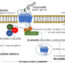
Fig. 1. The structure of PAH – The 2.9 Å PAH crystal structure is show in orthogonal views; each individual protein chain is colored by domain. The four chains are shown using four different molecular representations, which are ribbons, a Cα trace, sticks, and transparent spheres. In cyan, the chains are labeled near the catalytic domain (Top); in red, they are labeled near the regulatory domain (Bottom). The dotted black circle illustrates the autoregulatory region partially occluding the enzyme active site (iron, in orange sphere). (Arturo EC et al., Proc Natl Acad Sci U S A. 2016)
In 2013 Jaffe’s laboratory introduced an innovative new way to think about how PAH controls blood Phe levels. It takes into consideration the existence of very different shapes for PAH in a resting state and PAH in an activated state. In individuals with normal PAH, the amount of activated PAH increases after a protein-containing meal. This causes Phe to return to safe levels. Safe levels of Phe, in turn, cause PAH to return to its resting-state. This cycle is how a body normally responds to dietary protein. The published research reveals the shape of PAH in its resting state and how PAH moves to achieve an activated shape. The development of drugs that can control such molecular movements holds great promise for new PKU therapies.
Historically it has been difficult to pin down only one PAH enzyme shape and determine its molecular structure. Past work simplified the problem by studying parts of the PAH enzyme. PAH has three parts; by combining what is known about the shapes of the parts, the whole enzyme shape was approximated circa 1998. The 2016 publication reports how Jaffe’s team finally determined the crystal structure of full-length resting-state PAH and how this differs from the approximation. This long-awaited result is an important milestone in the PKU field. The crystal structure was determined by Emily Arturo of the Drexel University College of Medicine, under the supervision of Jaffe and Patrick J. Loll.
To understandboththe resting-state PAH shape and the activated PAH shape, Jaffe’s team used small angle X-ray scattering (SAXS) in collaboration with Emily Parker and Penelope Cross from University of Canterbury, New Zealand, as well as with Kushol Gupta of UPENN’s Perelman School of Medicine. The SAXS data, which reports on solution structure, is a good match to the crystal structure of resting-state PAH and is consistent with Jaffe’s 2013 prediction of the shape of activated PAH. Although this gives a birds-eye view of how PAH works to control Phe levels, molecular details about activated PAH remain unknown. Such details will provide an unprecedented basis for the design of new PKU drugs.
Research on the structure of activated PAH is continues, although complicated by limited support for fundamental research on the structural bases of orphan diseases. Such fundamental research, essential to the next generation of therapeutics, often occurs many years before clinical application.
Eileen K. Jaffe
Molecular Therapeutics, Fox Chase Cancer Center, Temple University Health Systems,
Philadelphia, PA, USA
Publication
First structure of full-length mammalian phenylalanine hydroxylase reveals the architecture of an autoinhibited tetramer.
Arturo EC, Gupta K, Héroux A, Stith L, Cross PJ, Parker EJ, Loll PJ, Jaffe EK.
Proc Natl Acad Sci U S A. 2016 Mar 1











Leave a Reply
You must belogged into post a comment.