A benevolent anionic ZnII-MOF to purify water and make medicines
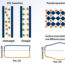
Metal-organic frameworks (MOF) are well-known crystalline porous materials comprising metal ions or metal clusters bridged by different types of organic linkers. MOFs have been in the spotlight globally owing to their tunable surface area, functionalizable pore surface, high chemical and thermal stability for which they find potential applications in gas storage and separation, catalysis, molecular sensing, drug delivery and in opto-electronic devices. MOFs are much superior when compared to other contemporary porous materials like zeolites or activated carbon in terms of modularity. We have synthesized a MOF {[Zn3(L)2][(NH2Me2)2].9H2O} (1) based on the self-assembly of Zn2+and a tetracarboxylate ligand (L), which is anionic in nature and has a guest dimethyl amine (NH2Me2+) (DMA) cation in its pores which maintains the charge neutrality. This cation can be exchanged to incorporate other metal cations or cationic species in the MOF 1. Water pollution from various noxious industrial wastes is one of the major environmental concerns. Constant exposure to such heavy metals causes deadly diseases like Minamata (Mercury), Plumbism (Lead) and Itai itai (Cadmium). Minamata disease impairs vital brain functions such as speech, sight, etc., whereas Plumbism is characterized by acute gastrointestinal problems and Itai itai causes brittle and weak bones. Since 1 is extremely water stable, we can use its cation exchanging property to capture toxic heavy metal cations like Mercury (Hg2+), Cadmium (Cd2+) and Lead (Pb2+). Upon investigating this property, it was found that 1 is capable of removing these heavy metal cations from water, even when their concentration is as low as 1 ppm, with an efficiency of nearly 98%. Thus, a combination of excellent water stability and high heavy metal removing efficiency implies that 1 holds a great potential to be used in practical purposes for removing heavy metals from toxic industrial effluents.
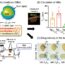
Next, we found that 1 is also capable of sequestering transition metals like Cu2+, Co2+and Ni2+. We wanted to explore the prospects of Cu2+encapsulated 1 as a catalyst. For this purpose, copper cation (Cu2+毛孔内的了1 by cation exchange. We used the resultant material as a catalyst for the room temperature synthesis of industrially important benzimidazole compounds, using a series of different substrates. The incorporated copper ion acts as a Lewis acid catalyst thereby making the reaction fast, lowering the reaction temperature and producing high yields of the benzimidazole compounds. Benzimidazoles are extremely important for their wide use for medicinal purposes and are active against various fungi, virus and carcinoma and also act as antihypertensive and antihistamine agents.
Hence, we have synthesized a ZnIIbased MOF, which has very significant dual activities of preventing health risks and assisting the synthesis of new medicinal compounds. On one hand, it is a new material to remove poisonous heavy metal cations from water, thereby preventing multiple health hazards. On the other, it acts as an excellent catalyst in the reaction leading to the synthesis of benzimidazole compounds, which have tremendous importance for their medicinal value.
Tapas K. Maji
Molecular Materials Lab, Chemistry and Physics of Materials Unit,
Jawaharlal Nehru Centre for Advanced Scientific Research, India
Publication
Post-synthetic metalation in an anionic MOF for efficient catalytic activity and removal of heavy metal ions from aqueous solution.
Chakraborty A, Bhattacharyya S, Hazra A, Ghosh AC, Maji TK
Chem Commun (Camb). 2016 Feb 4









Leave a Reply
You must belogged into post a comment.