Towards better tissue regeneration: Improved vessel growth using synthetic polymers
The formation of vascular network is crucial for development and function of most of the tissues due to oxygen and nutrients supply and removal of toxic metabolites. In case of an injury such as bone or soft tissue damage, tissue regeneration takes place where not only specific cells of particular tissue are being replaced but also vascular, lymphatic and neural connections must be recovered. Human body has only limited regenerative capacity therefore different approaches how to aid regenerative processes have been widely investigated and tested using a variety of materials, proteins and even cells. However, achieving functional vascularization of a graft still remains a challenge.

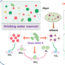
Fig. 1. PEA – fibronectin – VEGF constructs promoting vasculogenic response; a) Components of the system; b) Endothelial cells stimulated towards network formation in vitro, and new tissue formation inside a scaffold pore in vivo (endothelial cells in red, nuclei in cyan); c) Scheme of synergistic effect of integrin/growth factor receptor stimulation.
Our lab developed a specific platform to promote vascularization across a broad range of applications. To engineer our system stimulating vessel formation, three basic components are needed; a synthetic polymer poly(ethyl acrylate) (PEA), and two purified proteins, the extracellular matrix protein fibronectin (FN), and vascular endothelial growth factor (VEGF) (Fig. 1a). Physiologically, fibronectin facilitates a crosstalk between cells and extracellular space because its molecular structure provides several specific binding sites. Two types of binding sites important for our system are integrin binding domains where cells can attach, and growth factor (GF) binding domains which allow immobilization of GFs in extracellular space so they can interact with cell receptors to trigger cell signalling.
PEA is unique in promoting unfolding and organization of FN into nanonetworks where stretched FN fibrils similar to the physiological ones keep cell and GF binding domains simultaneously exposed and thus available for both, interaction with cells and sequestering GF. The fact that these two domains are in close vicinity is another advantage as it has been found that when FN-integrin binding and GF receptor activation happen close to each other, it has a synergistic effect on cellular stimulation, and a lower dose of a GF is needed to achieve similar cellular response in comparison to supplying soluble GF in the medium. This system has been already successfully tested by our group for using another GF, bone morphogenetic protein 2 (BMP-2) towards improved osteogenic differentiation of stem cells.
In this work we tested whether specific binding of VEGF to FN assembled on PEA has a stimulatory effect on vascularization events bothin vitroandin vivo. The vasculogenic response of human endothelial cells seeded on these synergistic interfaces was significantly improved. Not only the cells were forming better networks but also early onset of VEGF signalling (PLCγ phosphorylation) and common integrin and VEGF signalling (ERK1/2 phosphorylation) were increased when VEGF was bound to FN nanonetworks on PEA (Fig. 1b). We also observed co-localisation of αv integrin and VEGF receptor in cells on PEA functionalized with FN and VEGF that suggests integrin/growth factor receptor crosstalk. Experiments with mutant FN molecules with impaired integrin binding site (FN-RGE) confirmed an important role of the integrin binding domain of FN on the vasculogenic response via combined integrin/VEGF signalling. In vivo experiments using 3D scaffolds coated with FN and VEGF demonstrated pro-vascularization signalling by enhanced formation of new tissue inside scaffold pores (Fig. 1b).
Advantages of our engineered microenvironments above other systems helping vascularization and tissue regeneration in general that have been or are being developed are particularly its simple manufacturing, very low doses of growth factors needed for cell response due to synergistic effect with integrins (Fig.1c), and the fact that it is an acellular approach, with no need to incorporate cells. This makes our system very versatile as it can be used as a coating for different 2D and 3D devices and scaffolds for different regenerative medicine applications.
Vladimira Moulisova, Manuel Salmeron-Sanchez
MiMe Research Group, Division of Biomedical Engineering, School of Engineering,
University of Glasgow, Glasgow G12 8LT, United Kingdom
Publication
Engineered microenvironments for synergistic VEGF – Integrin signalling during vascularization.
Moulisová V, Gonzalez-García C, Cantini M, Rodrigo-Navarro A, Weaver J, Costell M, Sabater I Serra R, Dalby MJ, García AJ, Salmerón-Sánchez M
Biomaterials. 2017 May












Leave a Reply
You must belogged into post a comment.