辛迪思(IV)复杂的催化剂钛ize urea, thio-urea and guanidine
Well – defined organometallic complexes have played a pivotal role in homogeneous catalysis and in the development of environmentally benign processes. A number of transition and lanthanide metal complexes have already proven to be excellent catalysts in the formation of C-N bond via hydoamination reaction. Guanidine are commonly employed for several purposes. They can serve as building blocks in various pharmaceutical and natural products. They act as organic bases and catalyse various organic transformations.
They are also used as ancillary ligands in the preparation of a variety of metal complexes including those of main, transition and lanthanides metals. Urea and thio-urea functional groups also perform significant roles in organic, medicinal, supramolecular, and material chemistry. Earlier, main group metal complexes, such as LiN(SiMe3)2、烷基铝(Al (NMe2)3]2and magnesium metal complexes and variety of transition metal complexes such as Zn and V imido complexes have been utilized for the synthesis of guanidine and urea. In addition, commercially available alkyl metal complexes ZnEt2, MgBu2, n-BuLi, AlR3, and Zn(OTf)2have also been explored to be the efficient catalysts for this reaction. Titanium being the more abundant, in-expensive and relatively low toxic metal, can be potentially useful for this purpose. Further, its bio-affinity makes it suitable for innumerable applications in chemical synthesis.

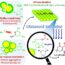
Fig. 2. A. Hydroamination of a number of amines with carbodiimides, isocyanates, and isothiocyanates using catalyst 1.
B. Plot of Kobs vs. [Catalyst 1)] for the reaction of constant concentration of 4-chlorophenylisocyanate and amine.
Jayeeta Bhattacharjee, Tarun K Panda
部门Chemistry, Indian Institute of Technology Hyderabad (IITH), Kandi, Telangana, India
Publication
Hydroamination of carbodiimides, isocyanates, and isothiocyanates by a bis(phosphinoselenoic amide) supported titanium(iv) complex.
Bhattacharjee J, Das S, Kottalanka RK, Panda TK
Dalton Trans. 2016 Nov 28










Leave a Reply
You must belogged into post a comment.