Stevensite: an efficient clay to retain tetracycline from wastewater
The tetracycline family of antibiotics are widely used for human therapy and animal feed. However, tetracycline (TC) is considered a contaminant of emerging concern in the environment due to its presence in wastewater effluents, surface waters and groundwaters. Conventional wastewater treatments, based on activated sludge processes, have shown limited capability of removing these pharmaceuticals from water. Additional treatments under study consist on the adsorption of TC on inert materials such as activated carbon, biochar, metallic oxides, ion exchange resins, soils and clays. A decreasing adsorption as the pH increases is normally reported in the pH range 3-9. This is disadvantageous since most of the municipal wastewaters are in a neutral to basic pH range.
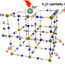
Stevensite is a type of smectite clay mineral with large surface area and cation exchange capacity. Adsorption isotherms of TC performed on a commercial stevensite clay with low presence of accessory minerals (<10% illite and dolomite, and <1% feldspar and quartz) presented promising results since adsorption increased with the increasing pH, in the pH range 2-8.
A laboratory design of a porous geofilter using a mixture of 90% sand and 10% stevensite was tested against an inflow solution of tetracycline 1 g/L, NaNO30.1 M and pH = 7 in an advective transport cell experiment. The geofilter was trapped in a cylindrical methacrylate column surrounded by a series of filters, including sintered stainless steel, porous Teflon, polypropylene geotextile and a 0.45 mm pore size polypropylene filter at both, inlet and outlet, sides.
A blank experiment, without stevensite, showed null adsorption of TC on filters and sand. When stevensite was used, the number of tetracycline molecules adsorbed exceed by more than 3 times the number exchangeable positions of stevensite. Under these conditions, the TC adsorption on the geofilter reached 590 mg/g, surpassing the retention capacity of most adsorbents found in literature. Besides, the TC was completely desorbed by the inflow of a saline solution (Mg(NO3)20.5 M, at pH = 2) with capacity to replace the exchangeable positions, thus, recovering both, the geofilter for its future use in additional cycles of TC adsorption, and the residual TC.
The analytical determinations confirmed no mineralogical or chemical transformations other than microstructural changes in layering staking due to TC adsorption on stevensite, thus, creating a process of disordered aggregation in the smectite layered structures and pores occlusion that decreased the mineral surface area. A mechanism of delamination and formation of complex structures of exchangeable divalent Mg and Ca cations in the binding process with TC was proposed to explain the excess of TC molecules retained.
The results obtained in our study are promising to implement a cost-effective design of TC retention in wastewater treatment plants. Future investigations will be focused on the stabilization of stevensite in the geofilter since its very small particle size may produce its own displacement out of the retention system with the infiltrating solution.
Raúl Fernández1,Ana Isabel Ruiz1,Carlos García-Delgado2,Daniel González-Santamaría1,Rafael Antón-Herrero2,Felipe Yunta2,Claudia Poyo1,Andrea Hernández1,Enrique Eymar2,Jaime Cuevas1
1Department of Geology and Geochemistry, Faculty of Sciences, Autonomous University of Madrid, Cantoblanco, 28049 Madrid, Spain
2Department of Agricultural Chemistry and Food Sciences, Faculty of Sciences, Autonomous University of Madrid, Cantoblanco, 28049 Madrid, Spain
Publication
Stevensite-based geofilter for the retention of tetracycline from water.
Fernández R, Ruiz AI, García-Delgado C, González-Santamaría DE, Antón-Herrero R, Yunta F, Poyo C, Hernández A, Eymar E, Cuevas J
Sci Total Environ. 2018 Dec 15












Leave a Reply
You must belogged into post a comment.