New ligand for preconcentration of lanthanides from environmental samples prior to ICP-MS analysis
Lanthanides are a group of chemical elements (La-Lu) with very similar behavior. Due to their unique properties they find application in many high-tech fields as computers, liquid crystal displays (LCDs), clinical MRI, lasers etc. The increased usage of these metals lead to their anthropogenic accumulation in the environment which increases their concentrations above the natural abundances. For these reasons determination of lanthanides in objects as waters, plants and soils is an important ecological issue.

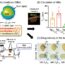
Fig. 1. Scheme of extraction procedure for liquid-liquid extraction of lanthanides with enaminone and ICP-MS determination.
Most commonly, lanthanides are at sub-ppb concentrations in environmental samples, so a very sensitive and selective method is needed for their determination. Despite the undisputed advantages of Inductively Coupled Plasma Mass Spectrometry (ICP-MS) for determination of these elements at sub-ppb levels, the analysis is strongly hindered in presence of barium which is a macro-component in waters and plants. To overcome this limitation, a method for preliminary separation and concentration of lanthanides is often required.
Compounds incorporating conjugated system N–C=C–C=O in their structure are called beta-enaminones and can be subdivided into beta-enaminoketones, beta-enaminoesters and beta-enaminoamides. This class of compounds has attracted our attention because of their potential to form chelating complexes with metal ions. Several researchers have published studies on the potential of enaminone compounds to form stable complexes with lanthanide ions.
In a recent study published in the scientific journal Talanta (vol. 160, 1 November, 2016, pp 389-399) we showed the applicability of one particular compound of the enaminones class, namely 3-ethylamino-but-2-enoic acid phenylamine, for separation and preconcentration of lanthanides from natural waters and plants. The scheme of the optimized extraction procedure in combination with ICP-MS as measurement technique is presented on Figure 1.
All parameters influencing extraction efficiency are optimized. It was established that the target reaction proceeds negligible in acidic and strong basic media, probably due to destruction of the ligand and hydrolysis of the metals respectively. Extraction optimum was obtained at pH=8, which is most probably caused by the enaminone protonation. The highest extraction degrees were obtained with 60 mg of enaminone ligand applying chloroform as extractant. Variation of solvent’s volume showed that minimum 5 mL chloroform is needed for separation of 100 µg L-1metals in 40 mL solution. The reaction proceeds very fast as 2 minutes are enough for quantitative extraction of the elements.
ICP has very low tolerance to organic solvents and thus analytes should be re-extracted in water phase before the measurement. The re-extraction was successfully completed with 1 mol L-1nitric acid for 2 minutes.
The optimized extraction procedure ensures efficient separation of lanthanides from barium and thus avoiding the spectral interferences during the ICP-MS measurements. The new ligand shows high selectivity towards alkali and alkaline-earth elements. Preconcentration factor of 10 provides decrease of method’s limits of quantification which were in the interval 0.7-12 ng L-1.
LLE-ICP-MS方法基于局域网的反应thanides with the new enaminone ligand was validated by analysis of certified reference material CCM NCS DC 73348 (Bush Branches and Leaves). The good agreement between the certified and determined values is a proof for the accuracy of the developed procedure. The method was successfully applied for determination of lanthanides in real water and plant samples.
E. Varbanova, P. Angelov, V. Stefanova
University of Plovdiv “Paisii Hilendarski”, Plovdiv, Bulgaria
Publication
Study of 3-Ethylamino-but-2-enoic acid phenylamide as a new ligand for preconcentration of lanthanides from aqueous media by liquid-liquid extraction prior to ICP-MS analysis.
Varbanova EK, Angelov PA, Stefanova VM
Talanta. 2016 Nov 1











Leave a Reply
You must belogged into post a comment.