Nanoparticle can directly permeate across cell membrane without membrane disruption
Nanoparticles (NPs) have been attracting much attention for biomedical and pharmaceutical applications. In most of the applications, NPs are required to translocate across the cell membrane and to reach the cell cytosol. Experimental studies have reported that by applying an electric field NPs can directly permeate across the cell membrane without the confinement of NPs by endocytic vesicles. However, damage to the cell can often be a concern. Understanding of the mechanism underlying the direct permeation of NPs under an external electric field can greatly contribute to the realization of a technology for the direct delivery of NPs. Here we investigated the permeation of a cationic gold NP across a phospholipid bilayer under an external electric field using a coarse-grained molecular dynamics simulation.

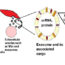
Fig. 1. Snapshots of cross-sectional side view and top view of nanoparticle (NP)-cell membrane interactions. Snapshots of cross-sectional side views of typical NP behaviors without the external electric field in (A) unbiased and (B) biased (pull) simulations. No permeation was observed and the NP adhered onto the membrane or was wrapped by the membrane. When an external electric field (V) that is equal to the membrane breakdown intensity (Vc) was applied (V = Vc), a typical NP delivery by electroporation was shown (D): the cationic gold NP directly permeated across a lipid bilayer without membrane wrapping of the NP, while a persistent transmembrane pore was formed. However, when a specific range of the electric field that is lower than the membrane breakdown intensity was applied (V < Vc), a unique permeation pathway was exhibited: the generated transmembrane pore immediately resealed after the direct permeation of NP (C). These results suggests that by applying an electric field in a suitable range NPs can be directly delivered into the cell with less cellular damage
We preliminarily investigated NP behavior under no external electric field (Fig. 1A and B). We have confirmed that without the external electric field the NP did not permeate across any lipid bilayer as shown below (Fig. 1A). Even when the NP was forced to move downward across the membrane under (i.e., the “pull” simulation) no external electric field, no permeation was observed and the NP was wrapped by the membrane (Fig. 1B).
In typical electroporation treatments, a somewhat excessive electric field that is higher than the membrane breakdown intensity is usually applied so that transient membrane defects can be formed in advance before delivering extracellular materials. Thus, we firstly conducted a MD simulation under an excessive electric field that is equal to the membrane breakdown intensity (Fig. 1D). In this case, a typical NP delivery by electroporation was shown: the cationic gold NP directly permeated across a lipid bilayer without membrane wrapping of the NP, while a persistent transmembrane pore was formed.
However, interestingly, we observed a drastic change when the external electric field was changed (Fig. 1C). When a specific range of the electric field that is lower than the membrane breakdown intensity was applied, a unique permeation pathway was exhibited: the generated transmembrane pore immediately resealed after the direct permeation of NP (Fig. 1C). Furthermore, we found that the affinity of the NP for the membrane surface is a key for the self-resealing of the pore.
These results strongly indicate that by controlling the external electric field as well as the NP surface properties in a suitable range, an ideal NP delivery pathway where the NP can be directly delivered into the cell with high delivery efficacy and less cellular damage can be achieved. Our finding in this study will contribute to the development of new technology for the direct delivery of NPs into cells with high efficacy and less cellular damage.
Hideya Nakamura
化学工程系,大阪公关efecture University, Japan
Publication
MD simulation study of direct permeation of a nanoparticle across the cell membrane under an external electric field.
Shimizu K, Nakamura H, Watano S
Nanoscale. 2016 Jun 9









Leave a Reply
You must belogged into post a comment.