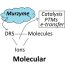
Dihydrogen phosphate ion comes at the guest to pillar[5]arenes
Selective anions’ binding is one of the key problems frequently occurred in design of supramolecular systems, in catalysis and material sciences as well as in some analytical applications (sensors, extraction systems, etc.). Among others, recognition of tetrahedral oxoanions is significant importance. Many of them are considered as environmentally dangerous (phosphates, sulfates) and hence require removal from industrial and agricultural wastewaters. Sensing phosphates and their derivatives is of special attention because they compose backbone of nucleic acids and play significant role in biological systems. Sensors for phosphate anions have been recently reported but they still need receptors with improved selectivity. Besides, H-bond receptors that show selectivity toward dihydrogen phosphate over acetate or fluoride anions are very scarce.
Pillar[5]arenes are the polyfunctional macrocyclic compounds consisting of 1,4-hydroquinone fragments coupled by methylene bridges in a macrocycle and having ten hydroxyl groups which can be easily modified. Thus, functionalization of pillar[5]arene allows carrying out synthesis of a macrocyclic host with desired properties. Importantly, hydrogen bonding is spatially sensitive, as it is formed only if the H-bond donor is positioned accurately in respect to the H-bond acceptor. This feature allows designing receptors with high selectivity toward anions that match the size and shape of the binding pocket. As a result, optimization of the “rigidity” of the host skeleton and introduction of additional functional groups becomes important. Thus, in this paper the line new amide-functionalized pillar[5]arenes1–3has been synthesized for decision about twin goals “binding strength – selectivity” (Fig. 1). Anions with various size and shape have been used to determine binding capability of1–3(Fig. 1). Addition of the fluoride, dihydrogen phosphate and acetate anions to solution of1resulted in a bathochromic (4.5 nm) and minor hyperchromic shift in the absorption maxima of the macrocycle1在240纳米。更多的突然变化被观察到in the case of interacting with dihydrogen phosphate ion. In the case of the pillar[5]arenes2and3, changes in the electronic spectra were found only in the presence of H2PO4 –indicating absence of interaction between the macrocycles and other anions. The measured association constants between1and F–, H2PO4 –and AcO–was the highest. The binding strength between1and F–is the strongest, and decrease gradually (F–> H2PO4 –> AcO–). However, pillar[5]arenes withN-aliphatic amide fragments are most selective macrocycles because they bind only dihydrogen phosphate anion. Therefore, the amide1is less selective than2and3, which form complex only with H2PO4 –. These results suggest two types of anion-binding centers in the macrocycle plane (NH and C=O). The H2PO4 –binding with the macrocycles1–3is obviously due to the proton accepting carbonyl group (H-bonds C=O·····HO-P) in the structure of these pillar[5]arenes, which is capable to selective recognition of dihydrogen phosphate anion. Besides, the ligands have ten pairs of the NH protons that can form strong hydrogen bonds with negatively charged oxygen atoms (H-bonds NH·····–O-P). The combination of these two factors has allowed creating effective and selective receptor for dihydrogen phosphate anion. The interaction of the anion with the macrocycle is due to hydrogen bonding of NH protons in macrocycle. Based on above described results for host/guest systems, we can assume the structures of the complexes formed (Fig. 2).So, these novel pillar[5]arene-based neutral anion receptors enrich structural diversity of anion binding chemistry, and can be further used in the fabrication of sensing devices for the dihydrogen phosphate anion.
Luidmila S. Yakimova, Dmitry N. Shurpik, Ivan I. Stoikov
Kazan Federal University, A.M. Butlerov Chemical Institute,
Kazan, Russian Federation
Publication
Amide-functionalized pillar[5]arenes as a novel class of macrocyclic receptors for the sensing of H2PO4- anion.
Yakimova LS, Shurpik DN, Stoikov II
Chem Commun (Camb). 2016 Oct 13











Leave a Reply
You must belogged into post a comment.