A new link between oxidant stress and lung cancer
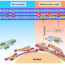
DNA repair protects our cells from conditions that cause damage to the genome, which can become a threat to the maintenance and function of our tissues. Our cells have a variety of DNA repair systems. Most frequent DNA lesion is damage to the base guanine by formation of “8-oxo-7,8-dihydroguanine (8-oxoG)”, and the DNA repair system that fixes this lesion is based on the base-excision enzyme 8-oxoguanine DNA glycosylase (OGG1). OGG1 performs two parallel functions: one the one hand it activates base-excision repair by binding to 8-oxoG, and on the other hand it activates inflammation by recruiting transcription factor NF-κB.
Inflammation becomes especially prevalent, when the DNA repair activity of OGG1 is inhibited by increases in oxidant stress. When oxidant stress in the tissue is very high, it inhibits OGG1 at least temporarily, allowing propagation of inflammation. In our lungs, when OGG1 switches to inflammation and delays its base-excision repair activity, it generates conditions that facilitate carcinogenesis, by progression of lung lesions to cancer.

Fig. 1. With increase in oxidant stress, the protein OGG1 enhances inflammation by recruiting NF-kB on DNA of inflammatory genes. A drop of oxidant stress allows OGG1 to initiate base-excision repair of DNA, which preserves DNA integrity and normal cellular function.
This becomes evident firstly because OGG1 recruits on the DNA the protein complex NF-κB that is a transcription factor that activates inflammation. Although NF-κB can have many effects in cells, OGG1 promotes NF-κB-driven inflammation. NF-κB-driven inflammatory gene expression is activated by the same mechanism as NF-κB-driven expression of genes that promote cancer progression. A key effect of this type of NF-κB-driven gene expression is that it inhibits the death of cancer cells, and their killing by the immune system. This type of NF-κB-driven gene expression requires NF-κB to interact with “Bromodomain and Extra-Terminal (BET)-proteins” to unwind the chromatin packaging of chromosome DNA. BET-proteins are already a target of lung cancer drugs under evaluation in clinical trials.
Furthermore, OGG1 facilitates the activity of the protooncogene product RAS, which is a known driver in lung cancer, also in the direction of higher NF-κB inflammatory activity. This excessively high inflammatory gene expression does not allow the immune system to function against the malignant tumor effectively. This happens because excessive expression of inflammatory genes activates several immunosuppressive mechanisms, which would normally serve to terminate an immune response. In lung cancer, immunosuppressive mechanisms predominate in the immediate vicinity of tumors and protect malignant cells, while in the rest of the tissue inflammation and activity of immune cells are exacerbated, leading to tissue damage.
Finally, mutations of OGG1 that can prolong the time that OGG1 activates NF-κB, have been associated with lung cancer. This type of mutations makes it longer until the OGG1 protein can resume DNA repair activity, when inactivated by oxidant stress. In contrast, the mutant enzyme can still recruit NF-κB on the DNA, and thereby cause high expression of genes that NF-κB regulates.
By analogy, another protein involved in DNA repair, namely PARP1, can also potentiate inflammatory NF-κB activity. In the case of PARP1, inhibitors are already approved for cancer treatment. PARP1 and OGG1 have complementary roles in DNA repair in cells. In vitro, eliminating OGG1 expression makes cells more sensitive to PARP1 inhibitors when exposed to oxidant stress.
Therefore, an inhibitor for OGG1-driven NF-κB activity could also become evaluated in the future in preclinical research, to develop future clinical trials for lung cancer. OGG1-driven NF-κB activity is partly inhibited by BET-protein blockers, which interfere with effects of the OGG1-driven NF-κB on chromatin. This type of blockers is already tested in clinical trials for solid tumors, which include lung cancer. In the future such blockers could be combined with inhibitors of OGG1 itself, to interfere specifically with cancer progression that is sparked by oxidant stress.
Spiros Vlahopoulos
Ηoremeio Research Laboratory, First Department of Paediatrics, National and Kapodistrian University of Athens, 11527 Athens, Greece
Publication
Roles of DNA repair enzyme OGG1 in innate immunity and its significance for lung cancer.
Vlahopoulos S, Adamaki M, Khoury N, Zoumpourlis V, Boldogh I
Pharmacol Ther. 2019 Feb









Leave a Reply
You must belogged into post a comment.