Using electrochemistry to address current needs in micro imaging
When one thinks of microscopy, usually imagined is something like a compound optical light microscope, which dates back before the 1600’s. For hundreds of years following, the optical microscope has served the scientific community by helping visualize cells, soil samples and other physical materials at the milli- and micro-scale. But what if we are interested in spatial organization of chemical groups, not their physical shape? The idea of “chemical imaging” tackles this question by identifying the chemical signature of different molecules, their concentrations and their location in space. This is the concept behind spectroscopic imaging and MRI, for example. In other applications, chemical images could be layered on top of optical images to find out where within a physical sample one would find different chemical groups.

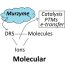
Fig. 1. (a) view of the software interface that displays raw data after various stages of data treatment and (b) an electrochemical image of a co-flowing stream of ferricyanide confined by two electrochemically inactive streams, superimposed on a design of the microfluidic channel used in the study.
Our group is interested in electrically active molecules, known as Redox molecules. “Reduction/oxidation” signifies a molecule’s ability to can gain or lose an electron when subjected to a voltage. For example, redox molecules such as adenosine triphosphate (ATP), hemoglobin and neurotransmitters, are central in the energy economy of cells, human respiration, and brain electrochemistry. Our interest stems from new energy sources called “microbial fuel cells”, where electroactive bacteria can liberate electrons during the oxidization of organic molecules found in wastewater.
To visualize redox molecules in microfluidics, we developedelectrochemical imaging(ECI). As a proof-of-principle we applied it to identify the location and dimensions of co-flowing streams of ferricyanide in a microchannel. When a ferricyanide (Fe(CN)63−) solution is beside an electrode, we can apply a voltage (electrical potential), which results in the addition of one electron to the molecule, thereby changing it via electrical charge reduction to a ferrocyanide (Fe(CN)64−) molecule:
Fe(CN)63−+ e−Fe(CN)64−E0=0.19V (1)
Where Fe is an atom of iron, C is carbon, N is nitrogen, e–是一个电子d E0is the reduction potential at which this reaction happens. Using a technique called “cyclic voltammetry” (CV), the voltage is gradually changed at an electrode surface while simultaneously monitoring the resulting current. When the voltage cycles past E0, we see a burst of current associated with the change of electronic state in (1). In electrochemical imaging we use a multiplexer to do this separately at every electrode in a high-density miniaturized electrode array. Our electrode array had 200 microscopic electrodes. To make the electrode arrays, we use a printed circuit board (PCB), the same found in your computer, due to low-cost and wide availability. Since we know the location of every electrode in the array, the signal generated at each one became an “electrochemical pixel”, which could be assembled into an electrochemical image. Figure 1 shows the custom software interface that controls the data acquisition, data treatment and image display. Using the same system we could also make streams of neurotransmitters like dopamine and serotonin or we could visualize the electrochemical reactions at the interface of different redox solutions. Our hope is that soon, anyone can easily image redox molecule concentrations in the confined spaces like a microchannel or virtually any other environment.
Jesse Greener
Département de Chimie, Université Laval, Québec, Canada
出版
Electrochemical imaging for microfluidics: a full-system approach.
Kara A, Reitz A, Mathault J, Mehou-Loko S, Amirdehi MA, Miled A, Greener J
Lab Chip. 2016 Mar 21








Leave a Reply
You must belogged into post a comment.