New materials challenge well-established theory of crystal growth
The classical theory of crystal growth was established over a century ago and is still used today to describe the formation of crystals with highly symmetrical shapes. This theory explains that crystals start as a single seed which then grows outwards, getting larger and larger over time as more atoms are deposited on its surfaces. The final shape of the crystal is simply dependent on the rate at which this deposition happens on different surfaces.

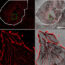
Fig.1. Crystals of zeolite analcime as they appear a) in nature, b) synthetically and c) crushed open, revealing the true inner structure.
In recent years, however, it has become apparent that this well-established theory cannot always explain how some crystals have formed. In 2007, a new mechanism was reported by Zhou and co-workers called “Reversed Crystal Growth”, which was first discovered in the zeolite analcime, which had been believed for many years to be single crystals, grown via the classical route, due to their perfect polyhedral outer appearance (Fig. 1.). When researchers crushed the crystals open, however, they found that it was actually made up of many nanocrystallites encased in a thin single crystal shell (Fig. 1c.). To work out how this interesting structure had formed, they studied the growth over time and showed that, contrary to the classical theory, the nanocrystallites first aggregated into some large micro-spheres instead of continuously growing as free crystals. The surface of these aggregates then recrystallised to form the single crystal layer while the inside remained disordered (i.e. a core-shell structure was formed). Eventually, the crystallisation extended from the surface inwards to the core.
In the present work, the growth mechanism of the material RHO-ZIF (RHO-type Zeolitic Imidazolate Framework) is studied, which is a very new and interesting material with a wide range of potential applications from catalysis to drug delivery. Discovering the true mechanism by which a useful material like this forms is crucial as only by truly understanding its growth can we tailor its morphology and, therefore, maximise its desirable properties for its many applications.

Fig. 2. Images showing a) the outer shape of the RHO-ZIF crystals, b,c) the core-shell structure revealed when the crystals were crushed. The arrows indicate the outer shell which increased in thickness as the growth time was extended from b) 24 h to c) 7 days.
By using a range of techniques to study the RHO-ZIF particles over time it is revealed that it also followed the reversed crystal growth route. In the first stage of growth, precursor molecules/ions formed many disordered, spherical aggregates that joined together to give rise to highly porous structures in a rhombic dodecahedral shape (see Fig. 2a.). In the next stage, the surface of these structures recrystallised to form a thin layer of single crystal, covering the disordered core (Fig. 2 b,c.). As the growth time was extended, this layer grew thicker towards the core until, eventually, true single crystals were formed.
Once this true mechanism was identified it was possible to solve problems that had previously been reported for this material. For example, reports of the nitrogen uptake of RHO-ZIF crystals were much lower than theoretical predictions but it is now apparent that the theoretical calculations were based on single crystals of RHO-ZIF when, in reality, the crystals tested would have had a core-shell structure. By understanding the growth and allowing the crystals to complete their transition to true single crystals, the reported surface area was almost doubled and the nitrogen uptake maximised.
Katherine Self
Publication
Reversed Crystal Growth of RHO Zeolitic Imidazolate Framework (ZIF).
Self K, Telfer M, Greer HF, Zhou W.
Chemistry. 2015 Dec 21











Leave a Reply
You must belogged into post a comment.