Receptors talking: solo vs chorus
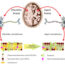
Multi-cell organisms, including humans, need reliable and timely communication between cells to function. Every cell is surrounded by the plasma membrane made of lipids, which separates it from the environment. Receptors localized on that membrane are proteins that serve as eyes and ears of the cell. They catch signals from the outside, such as hormones, neurotransmitters, nutrients, ions, etc., and translate them into the language the inside of the cell understands. We need to know the mechanism of this signal transduction to be able to fix things when they break down in disease.
Cells have many types of surface receptors. The most numerous group (~800 different subtypes in humans) are G protein-coupled receptors (GPCRs). GPCRs are responsible for our vision, sense of smell, taste, and many cell-to-cell communications in the body. This is why GPCRs are targeted by more clinically used drugs than any other protein family. Thus, knowing how GPCRs signal tells us how the drugs we use work and how new drugs should be designed to do a better job.
The majority of GPCRs appear to exist as monomers, but about a dozen types exist as complexes of two GPCRs, i.e., dimers. Some include two identical receptors (homo-dimers), whereas others are composed of two different molecules (hetero-dimers). Indirect evidence suggests that all GPCRs can form dimers with identical or different family members. This led to a hypothesis that GPCR dimerization is necessary for their signaling.
In the body every cell continuously receives many different signals. The response to a particular signal often depends on what other signals the cell receives at the same time. This is called signaling integration or signal cross-talk. Those who believe in dimerization of different GPCRs proposed that signaling integration starts right at the receptor level: a hetero-dimer can receive either one of the signals to which its components respond or both simultaneously, and that can determine the outcome. Others argued that there is a lot of cross-talk inside the cell between proteins that GPCR signaling activates or inhibits.
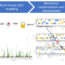
Three families of proteins directly bind activated GPCRs, and therefore can serve as signal transducers: G proteins, G protein-coupled receptor kinases, and arrestins. Many biochemical experiments performed by different labs over the years suggested that a single GPCR is necessary and sufficient to activate members of all three families. Recently the structures of GPCR complexes with G proteins, G protein-coupled receptor kinases, and arrestins were determined. These structures show GPCRs in the act of signaling. All complexes invariably contain a single GPCR interacting with each of these partners. As these new data agree with biochemical evidence, we can conclude that a single GPCR molecule mediates signaling.
然而,研究跟踪个人GPCR分子s show that on the cell surface these receptors exist in an equilibrium between monomeric and dimeric forms, both of which last less than a second before converting to the other. From experience biologists know that everything that exists in real life has a function. As overwhelming evidence shows that GPCR dimers are not needed for signaling, they must play a role in something else. Receptors have a complex life cycle. GPCRs are made inside the cell, then transported to the surface membrane, where they translate outside signals into cellular language, then removed from the surface membrane via the process called endocytosis, sorted inside the cell, and either returned to the surface or destroyed. Thus, it is reasonable to conclude that GPCR dimerization likely plays a role in their transport to the cell membrane or away from it, or in some other step in the complex life cycle of these receptors. The functional role of GPCR oligomerization still remains to be determined.
Vsevolod V. Gurevich, Eugenia V. Gurevich
Department of Pharmacology, Vanderbilt University, Nashville, TN 37232, USA
Publication
GPCRs and Signal Transducers: Interaction Stoichiometry.
Gurevich VV, Gurevich EV
Trends Pharmacol Sci. 2018 Jul









Leave a Reply
You must belogged into post a comment.