Production techniques for the development of sub-micron size polymeric drug delivery formulations evaluated
Polymeric drug delivery formulations containing active pharmaceutical ingredients (APIs) offer the advantages of improved stability, targeted delivery and controlled release of APIsin vivo,which can reduce drug burden while enhancing efficacy. Poly(lactic-co-glycolic acid) (PLGA) is amongst the most commonly studied materials for API delivery due to excellent biocompatibility, tuneable degradation characteristics and long clinical history of PLGA. Several applications of PLGA particles for the delivery of various APIs such as low molecular weight drugs, proteins, nucleic acids, vaccines have been reported within the past decades. When designed in sub-micron sizes, PLGA particles provide additional benefits such as potential for intravenous use. Therefore, therapies for various types of diseases may greatly benefit from clinical translation of sub-micron size PLGA formulations.

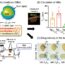
Fig. 1. Schemes of the batch method (1) and continuous process technologies (2, 3, 4) studied for the production of sub-micron size PLGA particles.
Despite the tremendous research emphasis put on the development of sub-micron size PLGA particles, no such formulation is currently available on the market. The clinical and commercial development of sub-micron size PLGA formulations is hampered by the challenges related to their good manufacturing practice (GMP)-compliant, scale-up production without affecting the formulation specifications. Lab-scale development of PLGA particles usually involve emulsion-based batch techniques, which are known to introduce modification of particle properties, including drug release profiles, upon scaling up the production. As subtle variations in the manufacturing process can significantly alter the product characteristics, alternative production techniques with ‘seamless’ scalability should be explored. In this respect, continuous processes offer the advantage of preferential termination of the production at the desired scale without changing the process parameters.
In our study, we evaluated three well-established process technologies for large-scale production of sub-micron size PLGA particles in comparison to a batch technique in terms of particle size distribution, throughput and GMP-compliance. We demonstrated the optimization of critical process and formulation parameters for high-pressure homogenization, high-shear mixing and microfluidics technologies (Fig. 1) to obtain PLGA particles with an average diameter of 150 nm – 250 nm and a small polydispersity index (PDI, ≤ 0.2).
Depending on the working principle of manufacturing devices, different parameters were found to affect the resulting particle size and polydispersity. The versatility of production methods allowed for fine-tuning of the formulation characteristics via optimizing process and formulation parameters. However, each method was associated with different drawbacks in terms of ease of material handling, aseptic production possibility and the cost of production. Table 1 shows the pros and cons of each technique.

Tab. 1. Comparison of pros, cons and throughput of techniques studied for the production of sub-micron size PLGA particles.
最终决定最合适的产品ion method would largely favor those that offer cost-effective, GMP-compliant and the least laborious production. In our study, high-shear mixing was found to be particularly promising due to the availability of GMP-ready equipment and large throughput of production. Overall, the results of our comparative study can be of great help in decision making towards GMP-compliant large scale production of sub-micron size PLGA particles, facilitating their commercial and clinical development.
Maria Camilla Operti, Carl G. Figdor, Oya Tagit
Department of Tumor Immunology, Radboud Institute for Molecular Life Sciences, Radboud University Medical Center, Nijmegen, The Netherlands
Publication
A comparative assessment of continuous production techniques to generate sub-micron size PLGA particles.
Operti MC, Fecher D, van Dinther EAW, Grimm S, Jaber R, Figdor CG, Tagit O
Int J Pharm. 2018 Oct 25








Leave a Reply
You must belogged into post a comment.