Overproduction of membrane-integral undecaprenyl pyrophosphate phosphatase from Vibrio vulnificus
In the cytoplasm, undecaprenyl pyrophosphate (C55-PP) is synthesized by undecaprenyl pyrophosphate synthase (UppS) through consecutive condensation reactions of eight molecules of isopentenyl pyrophosphate (Ipp) with farnesyl pyrophosphate (Fpp). The product C55-PP is then dephosphorylated to monophosphate undecaprenyl phosphate (C55-P) inde novosynthesis by an integral membrane protein, undecaprenyl pyrophosphate phosphatase (UppP). C55-P then serves as a carrier lipid for the translocation of hydrophilic oligosaccharide precursors (Lipid II) across the plasma membranes for peptidoglycan assembly and various carbohydrate polymers biosynthesis, such as lipopolysaccharides, teichoic acids, and osmoregulated periplasmic glucans.

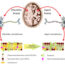
Fig.1. (a) Diagram of the process for purifyingV. vulnificusUppP. The N-terminal bacteriorhodopsin (purple) fused-UppP (green) complexes are over-expressed in theE. colicell membrane (yellow). After cell disruption, the n-Dodecyl β-D-maltoside (DDM) is used for extraction of proteins from the membranes, and solubilizes them by creating a mimic of the natural lipid bilayer environment. Protein-detergent complexes are then purified using Ni-NTA affinity chromatography for further biochemical and structural analyses. (b) Overall structural model ofV. vulnificusUppP in complex with C55-PP and Mg2+. Transmembrane helices, labeled from 1 to 8, are shown as cylinders and colored in rainbow. The extracellular helix is purple and labeled as H1. The C55-PP substrate is shown as grey for its carbon molecules. (c) RMSF of individual residues of the UppP-C55-PP complex in 30 ns of MD simulation.
The goal of the current studies is to overproduce the UppP homolog inVibrio vulnificus, and to determine its catalytic mechanism and structural information within the membrane compartment.V. vulnificus海洋病原体leading to rapidly expanding cellulitis or septicemia in humans, often causes large, disfiguring ulcers that require extensive debridement or amputation, making it an extremely virulent bacterium. As UppP catalyzes the production of C55-P during the biosynthesis of bacterial cell walls, this essential metabolic step is expected to be a potential target in searches for new antibiotics forV. vulnificus和其他细菌性病原体。
In this work, we purified activeV. vulnificusUppP using a bacteriorhodopsin as a tag fused at the N-terminus of the target proteins (Fig. 1a). Because this system opens new opportunities to investigate specific amino acids critical to enzymatic catalysis using site-directed mutagenesis, we determined the catalytic site of UppP which comprises two consensus motifs—a glutamate/serine (E/QGxxExLPxSSxxH) domain and a putative structural P-loop (PGxSRSGxT). In the former domain, the strictly conserved histidine residue could initiate a nucleophilic attack on the phosphorus center during the substrate hydrolysis reaction. Our structural model and molecular dynamics (MD) simulation studies also show that bacterial UppP has eight transmembrane helices (TMHs), for which the two consensus regions are localized near the aqueous interface oriented toward the periplasm, and both the N- and C-termini are located at the cytosolic site (Fig. 1b and 1c).
In summary, our biochemical and structural analyses suggest a plausible structure of the catalytic core, centered on two consensus regions that mediate the substrate dephosphorylation reaction at the periplasm instead of in the cytosolic compartment. Our data provided new insights into the molecular basis of the UppP-C55-PP interaction in membrane bilayers, and can be applied generally to all bacterial UppP enzymes.
Hsin-Yang Chang1,Chia-Cheng Chou2,Andrew H.-J. Wang3
1Department of Marine Biotechnology and Resources, National Sun Yat-sen University, Kaohsiung, Taiwan
2National Center for High Performance Computing, Hsinchu, Taiwan
3Institute of Biological Chemistry, Academia Sinica, Taipei, Taiwan
Publication
Expression, purification and enzymatic characterization of undecaprenyl pyrophosphate phosphatase from Vibrio vulnificus.
Chang HY, Chou CC, Wu ML, Wang AHJ
Protein Expr Purif. 2017 May











Leave a Reply
You must belogged into post a comment.