How to stain E-selectin ligands in tumor tissue
Cancer affects millions of people around the world. Nowadays they have more chances to survive, but still, the most frightful scenario are metastases. Few patients with metastases survive, and there is an unmet clinical need for new diagnosis and treatment methods.
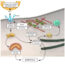
We know that cancer cells leave the original site, enter the bloodstream and migrate to a new distant site. Exiting the blood is not an easy job. If we imagine people being rescued from a rapidly flowing river, they need to approach the shore to be saved. Rescuing is largely facilitated by help on the shore. Similarly, in cancer, to exit the blood, only circulating cancer cells that have potent arms (ligands) can attach to receptors on the venous endothelial cells (the shore) and extravasate (rescue).
The immunohistochemical staining of E-selectin ligands (cells’ potent arms) is generally performed in standardized protocols using monoclonal antibodies that recognize their binding determinants, Sialyl Lewis x (sLex) and Sialyl Lewis a (sLea), such as the clone HECA-452. The aberrant expression of these epitopes by cancer cells is usually associated with cancer progression and high metastatic capacity. However, it is controversial whether these antibodies mimic E-selectin binding since the role of the scaffold protein decorated with such epitopes is also essential in E-selectin binding activity.
Here, we first tested and developed a novel staining protocol for paraffin-embedded cancer tissues using a mouse E-selectin-human Ig Fc chimaera (E-Ig), validated for the identification of E-selectin ligands in human cells.
Formalin-fixed paraffin-embedded tissues are sectioned and placed onto slides using standard paraffin microtomy. The “Lab Vision PreTreatment Module” (PTM) from Thermo Scientific, is used for deparaffinization, rehydration and heat-induced antigen retrieval (heating cycles) on tumour sections, in one step.
After blocking endogenous peroxidases (3% H2O2solution), slides are stained using a three-step procedure with the E-Ig (1:300), anti-CD62E (1:250) and HRP polymer (Fig. 1). The “HiDef Detection HRP Polymer System” detects the primary antibody by the antibody’s amplification (Amplifier) followed by HRP polymer (Detector). The chimeric molecule and anti-CD62E antibody dilution were prepared using “Diamond: Antibody Diluent”, which contains bovine serum albumin as blocking agent. Additionally, all the solutions in this staining protocol should have 2mM CaCl2, since the binding of E-selectin receptor to E-selectin ligands is calcium-dependent.

Fig. 2. Staining of colon adenocarcinoma (A and B) and normal colon (C and D) tissue with E-Ig (A and C) and HECA-452 (B and D). Brown color indicates E-Ig and HECA-452 positive reactivity. In cancer tissue, the lamina propria showed E-Ig staining exclusively on neoplastic cells, while HECA-452 staining showed positive scattered staining. In normal tissue, both E-Ig chimera and HECA-452 stains the lumens of the crypts, particularly in goblet cells. 40X magnification.
This strategy, using E-Ig, allowed successful immunohistochemical staining and was more specific for cancer cells, compared with anti-sLex/astaining. Moreover, staining was not detected when we used a calcium chelator as a control assay.
Therefore, both protocols, this and the one currently used, stain tumour tissues and normal goblet cells. However, the E-Ig chimaera staining generates more specific and clearer staining, while the staining of the currently used anti-sLex/aantibodies is more scattered and non-specific, mainly within the lamina propria (Fig. 2). This increased efficiency is related to direct staining in the ligands that effectively have the potential to bind to E-selectin, simulating the potential of these cells to metastasize.
Detection of neoplastic cells using this methodology was also effective in tissues of different types of cancer. Moreover, the protocol can be further adapted to other techniques, such as immunofluorescence, depending on the reporter system used in the final step.
我们相信这个染色技术将facilitate our understanding of the molecular basis of tumour progression and metastasis, providing us with a novel approach for immunohistochemical analysis of E-selectin ligands in cancer tissues.
A. Gonçalo Mineiro1,Paula A. Videira1,2
1UCIBIO-REQUIMTE, Department of Life Sciences, Faculty of Science and Technology, NOVA University of Lisbon, Lisbon, Portugal
2CDG & Allies – Professionals and Patient Associations International Network (CDG & Allies-PPAIN), Caparica, Portugal
Publication
Staining of E-selectin ligands on paraffin-embedded sections of tumor tissue.
Carrascal MA, Talina C, Borralho P, Gonçalo Mineiro A, Henriques AR, Pen C, Martins M, Braga S, Sackstein R, Videira PA
BMC Cancer. 2018 May 2












Leave a Reply
You must belogged into post a comment.