Building an artificial kidney from human stem cells
Many diseases and drugs affect kidney function by damaging the glomeruli — the major site of blood filtration and a target for many diseases. Anin vitromodel of the human glomerulus could facilitate therapeutic discovery and illuminate kidney disease mechanisms. Efforts to develop such models have been limited by the lack of a reliable source of functional human podocytes, the specialized epithelial cells that regulate selective permeability in the kidney glomerulus. Despite recent advances in generating nephron progenitors and multicellular organoid structures, a method for the differentiation of human induced pluripotent stem (hiPS) cells into functional podocytes with specificity and efficiency remains elusive.

图1所示。人类iPS细胞的定向分化s into mature kidney cells. (A) Schematic illustration of the method for human iPS cell differentiation into kidney glomerular podocytes. (B) Transcriptome analysis of genes involved in different stages of kidney development. Red, up-regulated; Blue, down-regulated. (C) Immunofluorescence staining showing the expression of podocin (green), a podocyte lineage identification marker, in the human iPS cell-derived kidney cells. (D) Scanning electron micrographs of human iPS cell-derived kidney podocytes compared to an established human podocyte cell line (PCL) which lacks morphological features necessary for kidney function.
To address these challenges, we developed a highly efficient method for directed differentiation of hiPS cells into functional kidney glomerular podocytes which was enabled by the discovery of key signaling molecules that support adhesion and stimulate gene regulatory pathways in human kidney development. The resulting hiPS cell-derived podocytes express markers consistent with mature phenotype, exhibit primary and secondary foot processes, and integrate into forming glomerular structures when injected into mouse embryonic kidney explants.
We leveraged the hiPS cell-derived podocytes and organs-on-chips technology to engineer anin vitromodel of a functional human kidney glomerular capillary wall. Unlike previously reported kidney organoids that lack fluid path or circuitry and have limited control over tissue structure and function, we found that our human glomerulus-on-a-chip supports podocyte differentiation and recapitulates the tissue-tissue interface (podocytes―basement membrane―endothelium) and selective molecular filtration function of the glomerulus. Our results demonstrate the feasibility of generating mature podocytes from unspecialized stem cells in a robust manner, providing a unique opportunity to engineer a functional human kidney glomerulus-on-a-chip. These results could facilitate investigations that aim to uncover the molecular and cellular mechanisms of human kidney development, function, and disease progression.
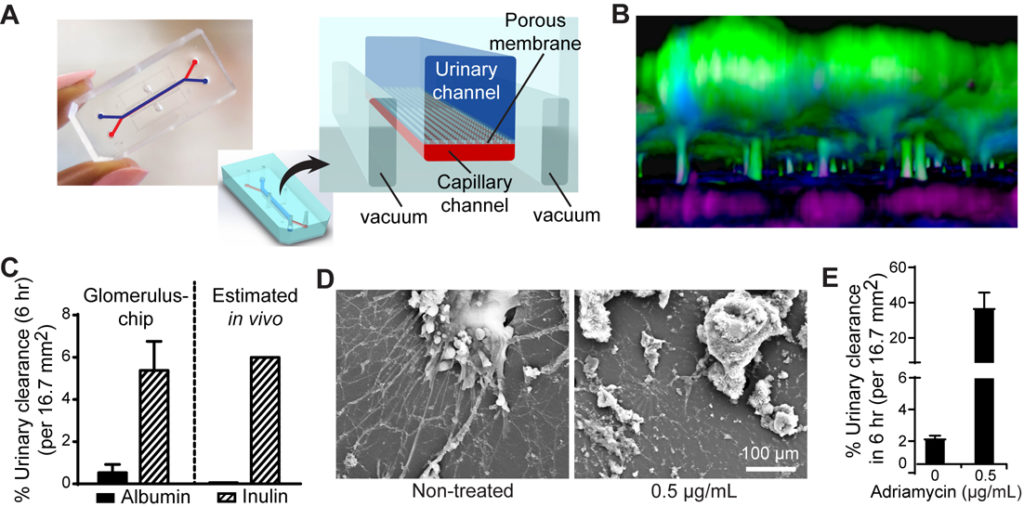
Fig. 2. Establishment of a functional human kidney glomerulus-on-a-chip. (A) Microfluidic organ-on-a-chip device showing dedicated compartments to mimic the vasculature (capillary channel) and podocyte layer (urinary channel). (B) 3D reconstruction of the human kidney’s blood filtration barrier modeled using iPS cell-derived podocytes (green) and primary glomerular endothelium (magenta). (C) Selective filtration of molecules in the engineered glomerulus-on-a-chip compared to an estimated in vivo values. (D) Modeling podocyte injury induced by the chemotherapy drug Adriamycin. Scanning electron micrographs showing damage to podocytes exposed to clinically relevant concentration of the drug. (E) Glomerulus-on-a-chip models adriamycin-induced albuminuria. Bar graphs represent the concentration of albumin in the urinary compartment or loss of albumin protein into urine, a hallmark of kidney disease.
These results also provide a low-cost alternative to animal models for nephrotoxicity testing, drug screening, and the development of novel therapeutic strategies for both genetic and acquired forms of human kidney diseases. Given that specialized podocytes in a fully formed human kidney are post-mitotic and unable to undergo regenerative proliferation to compensate for cell loss or an increase in glomerular basement membrane surface areain vivo, our results also provide opportunities for cell-based therapy and regenerative medicine approaches for kidney disease, including chronic kidney disease and podocytopathies.
Samira Musah
Department of Biomedical Engineering, Pratt School of Engineering, Duke University, Durham, NC, USA
Department of Medicine, Division of Nephrology, Duke University School of Medicine, Durham, NC, USA
Publications
Directed differentiation of human induced pluripotent stem cells into mature kidney podocytes and establishment of a Glomerulus Chip.
Samira Musah, Nikolaos Dimitrakakis, Diogo M. Camacho, George M. Church, Donald E. Ingber
Nat Protoc. 2018 Jul
Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip.
Musah S, Mammoto A, Ferrante TC, Jeanty SSF, Hirano-Kobayashi M, Mammoto T, Roberts K, Chung S, Novak R, Ingram M, Fatanat-Didar T, Koshy S, Weaver JC, Church GM, Ingber DE
Nat Biomed Eng. 2017









Leave a Reply
You must belogged into post a comment.